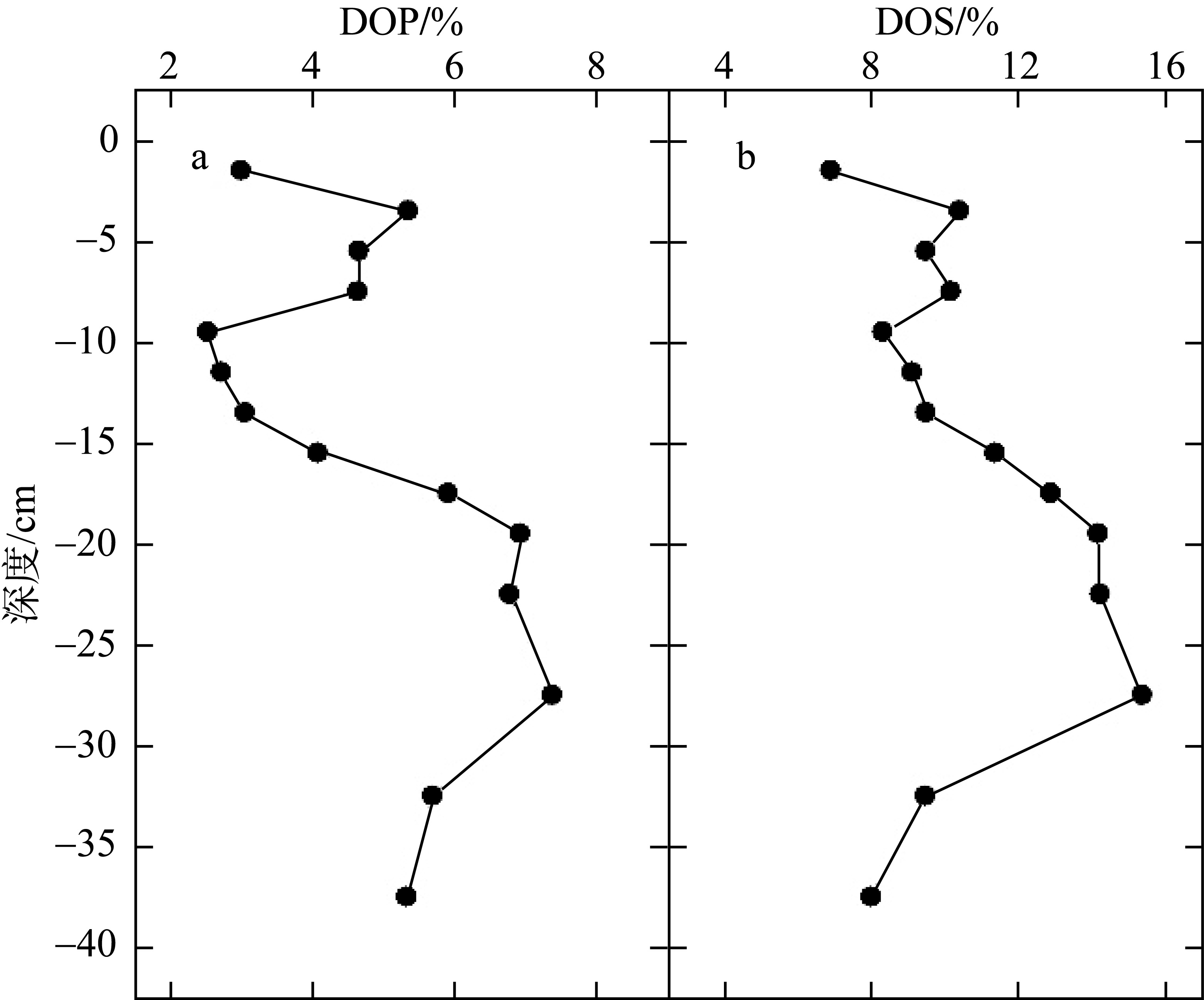
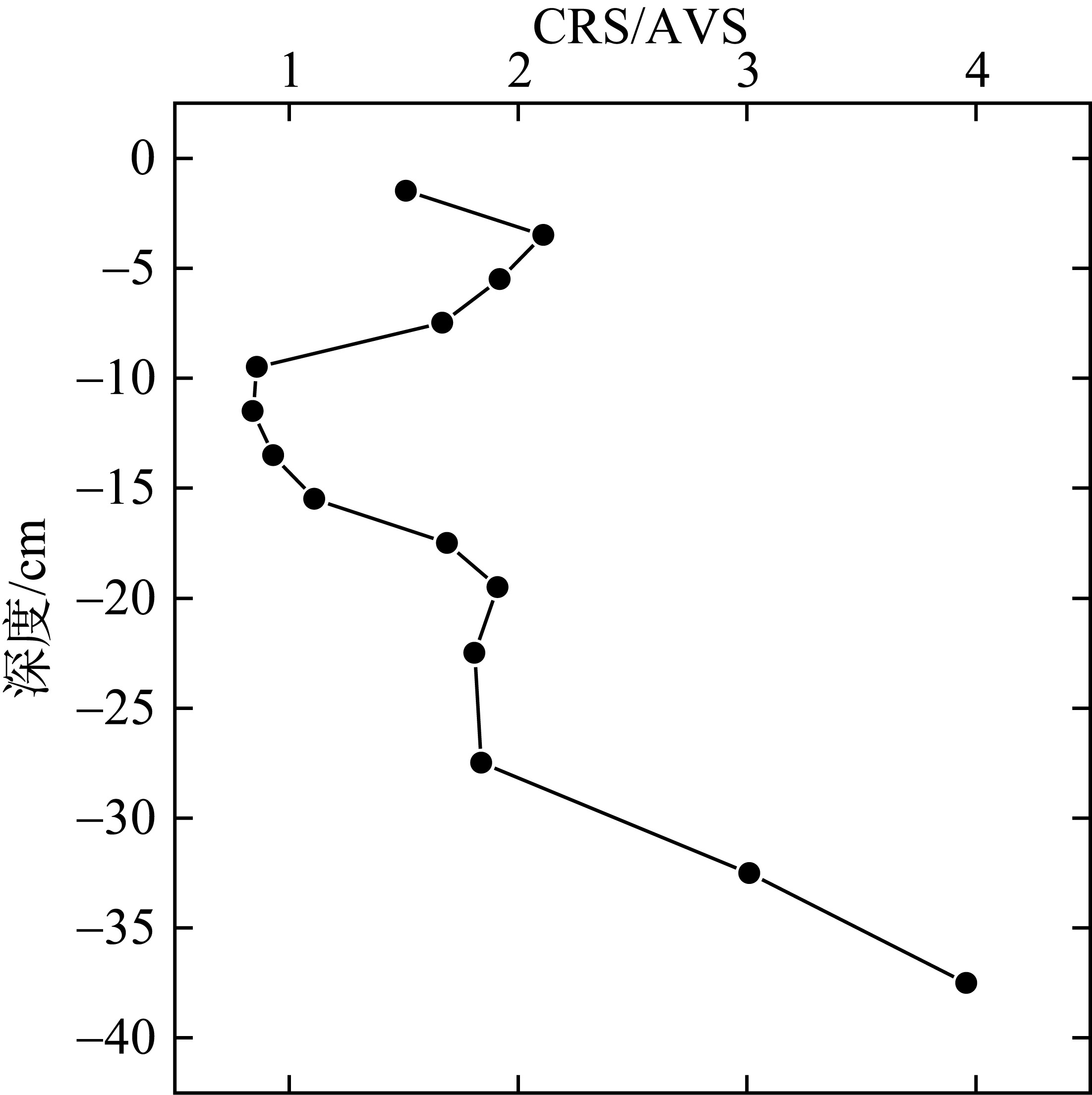
| [1] |
曹爱丽, 周桂平, 胡姝, 等, 2010. 崇明东滩湿地沉积物中还原无机硫的形态特征[J]. 复旦学报(自然科学版), 49(5): 612-617.
|
|
CAO AILI, ZHOU GUIPING, HU SHU, et al, 2010. The Chemical characteristic of reduced inorganic Sulfur in the Wetland sediments of Chongming Dongtan[J]. Journal of Fudan University (Natural Science), 49(5): 612-617 (in Chinese with English abstract).
|
| [2] |
丰卫华, 王志富, 张荣保, 等, 2016. 宁德海域表层沉积物氧化还原环境特征及其影响因素[J]. 海洋环境科学, 35(6): 882-887.
|
|
FENG WEIHUA, WANG ZHIFU, ZHANG RONGBAO, et al, 2016. The environment characteristics of redox in the surface sediments of Ningde coastal sea area in relation to influence factors[J]. Marine Environmental Sciences, 35(6): 882-887 (in Chinese with English abstract).
|
| [3] |
国家环境保护总局, 2002. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社: 368-370.
|
|
北京:中国环境科学出版社: 368-370. THE STATE ENVIRONMENTAL PROTECTION ADMINISTRATION, 2002. Water and wastewater monitoring and analysis method[M]. Fourth Edition. Beijing: China Environmental Science Press: 368-370 (in Chinese with English abstract).
|
| [4] |
黄巧义, 唐拴虎, 卢瑛, 等, 2014. 酸性硫酸盐土的形成、特性及其生态环境效应[J]. 植物营养与肥料学报, 20(6): 1534-1544.
|
|
HUANG QIAOYI, TANG SHUANHU, LU YING, et al, 2014. Formation, characteristics and ecological effects of acid sulfate soil[J]. Journal of Plant Nutrition and Fertilizer, 20(6): 1534-1544 (in Chinese with English abstract).
|
| [5] |
黄旭, 唐拴虎, 杨少海, 等, 2015. 酸性硫酸盐土壤改良对不同品种水稻生育性状的影响[J]. 中国土壤与肥料, (6): 48-56.
|
|
HUANG XU, TANG SHUANHU, YANG SHAOHAI, et al, 2015. Effects of acid sulphate soil improvement on growth characters of different varieties of rice[J]. Journal of Chinese soil and fertilizer, (6): 48-56 (in Chinese with English abstract).
|
| [6] |
姜明, 赵国强, 李兆冉, 等, 2018. 烟台夹河口外柱状沉积物还原性无机硫、活性铁的变化特征及其相互关系[J]. 海洋科学, 42(8): 90-97.
|
|
JIANG MING, ZHAO GUOQIANG, LI ZHAORAN, et al, 2018. Distribution characteristics and relationship between reduced inorganic sulfur and reactive iron in core sediments outside the mouth of the Jiahe River in Yantai[J]. Marine Sciences, 42(8): 90-97 (in Chinese with English abstract).
|
| [7] |
吕仁燕, 朱茂旭, 李铁, 等, 2011. 东海陆架泥质沉积物中固相Fe形态及其对有机质、Fe、S成岩路径的制约意义[J]. 地球化学, 40(4): 363-371.
|
|
LYU RENYAN, ZHU MAOXU, LI TIE, et al, 2011. Speciation of solid-phase iron in mud sediments collected from the shelf of the East China Sea: Constraints on diagenetic pathways of organic matter iron and sulfur[J]. Geochimica, 40(4): 363-371 (in Chinese with English abstract).
|
| [8] |
钱宝, 刘凌, 肖潇, 2011. 土壤有机质测定方法对比分析[J]. 河海大学学报(自然科学版), 39(1): 34-38.
|
|
QIAN BAO, LIU LING, XIAO XIAO, 2011. Comparative tests on different methods for content of soil organic matter[J]. Journal of Hohai University (Natural Sciences), 39(1): 34-38 (in Chinese with English abstract).
|
| [9] |
施玉珍, 张际标, 李雪英, 等, 2012. 湛江湾沉积物中酸可挥发性硫和重金属含量分布及重金属生态风险评价[J]. 应用海洋学学报, 31(4): 466-471.
|
|
SHI YUZHEN, ZHANG JIBIAO, LI XUEYING, et al, 2012. Distribution of acid-volatile sulfide (AVS) and heavy metal contents in sediment of Zhanjiang Bay and ecological risk assessment of heavy metals[J]. Applying the Journal of Oceanology, 31(4): 466-471 (in Chinese with English abstract).
|
| [10] |
孙启耀, 2016. 河口沉积物硫的地球化学特征及其与铁和磷的耦合机制初步研究[D]. 烟台: 中国科学院烟台海岸带研究所.
|
|
SUN QIYAO, 2016. Geochemical characteristics of sulfur and its coupling mechanism with iron and phosphorus in estuary sediments[D]. Yantai: Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences (in Chinese with English abstract).
|
| [11] |
唐罗忠, 生原喜久雄, 户田浩人, 等, 2005. 湿地林土壤的Fe2+, Eh及pH值的变化[J]. 生态学报, (1): 103-107.
|
|
TANG LUOZHONG, HAIBARA KIKUO, TODA HIROTO, et al, 2005. Dynamics of ferrous iron, redox potential and pH of forested wetland soils[J]. Acta ecologica sinica, (1): 103-107 (in Chinese with English abstract).
|
| [12] |
尹洪斌, 范成新, 丁士明, 等, 2008. 太湖梅梁湾与五里湖沉积物活性硫和重金属分布特征及相关性研究[J]. 环境科学, 29(7): 1791-1796.
|
|
YIN HONGBIN, FAN CHENGXIN, DING SHIMING, et al, 2008. Distribution Characteristic and Correlation Relationship of Reactive Sulfur and Heavy Metals in Sediments of Meiliang Bay and Wuli Lake of Taihu Lake[J]. Environmental science, 29(7): 1791-1796 (in Chinese with English abstract).
|
| [13] |
于雯泉, 钟少军, 蒲晓强, 等, 2009. 胶州湾李村河河口区沉积物有机碳、酸可挥发硫化物及重金属元素的环境响应[J]. 古地理学报, 11(3): 338-347.
|
|
YU WENQUAN, ZHONG SHAOJUN, PU XIAOQIANG, et al, 2009. Environmental responses of total organic carbon (TOC), acid volatile sulfide (AVS) and heavy metal elements in sediments of Licun Estuary in Jiaozhou Bay[J]. Journal of Palaeogeography, 11(3): 338-347 (in Chinese with English abstract).
|
| [14] |
张伯虎, 陈沈良, 刘焱雄, 等, 2011. 广西钦州湾海域表层沉积物分异特征与规律[J]. 热带海洋学报, 30(4): 66-70.
|
|
ZHANG BOHU, CHEN SHENLIANG, LIU YANXIONG, et al, 2011. Sediment characteristics and differentiation in the Qinzhou Bay, Guangxi, China[J]. Journal of Tropical Oceanography, 30(4): 66-70 (in Chinese with English abstract).
doi: 10.11978/j.issn.1009-5470.2011.04.066
|
| [15] |
章家恩, 1999. 酸性硫酸盐土的酸害暴发机制及其环境影响[J]. 热带地理, 19(2): 137-141.
|
|
ZHANG JIAEN, 1999. The Process of acid hazards in acid sulfate soils and its environmental effects[J]. Tropical Geography, 19(2): 137-141 (in Chinese with English abstract).
|
| [16] |
张璐, 2014. 胶州湾沉积物中硫酸盐还原和铁异化还原的影响因素研究[D]. 青岛: 中国海洋大学.
|
|
ZHANG LU, 2014. Study on factors influencing sulfate reduction and microbial iron reduction in Jiaozhou Bay sediments[D]. Qingdao: Ocean University of China (in Chinese with English abstract).
|
| [17] |
ARFAEINIA H, NABIPOUR I, OSTOVAR A, et al, 2016. Assessment of sediment quality based on acid-volatile sulfide and simultaneously extracted metals in heavily industrialized area of Asaluyeh, Persian Gulf: concentrations, spatial distributions, and sediment bioavailability toxicity[J]. Environmental Science and Pollution Research, 23(10): 9871-9890.
doi: 10.1007/s11356-016-6189-0
|
| [18] |
BENNING L G, WILKIN R T, BARNES H L, 2000. Reaction pathways in the Fe-S system below 100℃[J]. Chemical Geology, 167(1-2): 25-51.
doi: 10.1016/S0009-2541(99)00198-9
|
| [19] |
CHEN MEIQIN, LU GUINING, GUO CHULING, et al, 2015. Sulfate migration in a river affected by acid mine drainage from the Dabaoshan mining area, South China[J]. Chemosphere, 119: 734-743.
doi: S0045-6535(14)00978-3
pmid: 25189685
|
| [20] |
FABBRI D, LOCATELLI C, SNAPE C E, et al, 2001. Sulfur speciation in mercury-contaminated sediments of a coastal lagoon: the role of elemental sulfur[J]. Journal of Environmental Monitoring, 3(5): 483-486.
pmid: 11695115
|
| [21] |
FANNING D S, RABENHORST M C, BALDUFF D M, et al, 2010. An acid sulfate perspective on landscape seascape soil mineralogy in the U. S. Mid-Atlantic region[J]. Geoderma, 154(3-4): 457-464.
doi: 10.1016/j.geoderma.2009.04.015
|
| [22] |
FANNING D S, RABENHORST M C, FITZPATRICK R W, 2017. Historical developments in the understanding of acid sulfate soils[J]. Geoderma, 308: 191-206.
doi: 10.1016/j.geoderma.2017.07.006
|
| [23] |
FLORIAN S, SILKE S, JAMES M, et al, 2014. On the isotope composition of reactive iron in marine sediments: Redox shuttle versus early diagenesis[J]. Chemical Geology, 389: 48-59.
doi: 10.1016/j.chemgeo.2014.09.009
|
| [24] |
GAGNON C, MUCCI A, PELLETIER E, 1995. Anomalous accumulation of acid-volatile sulphides (AVS) in a coastal marine sediment, Saguenay Fjord, Canada[J]. Geochimica et Cosmochimica Acta, 59(13): 2663-2675.
doi: 10.1016/0016-7037(95)00163-T
|
| [25] |
HANSEL C M, LENTINI C J, TANG YUANZHI, et al, 2015. Dominance of sulfur-fueled iron oxide reduction in low-sulfate freshwater sediments[J]. The Isme Journal, 9(11): 2400-2412.
doi: 10.1038/ismej.2015.50
|
| [26] |
JOHNSTON S G, MORGAN B, BURTON E D, 2016. Legacy impacts of acid sulfate soil runoff on mangrove sediments: Reactive iron accumulation, altered sulfur cycling and trace metal enrichment[J]. Chemical Geology, 427(6): 43-53.
doi: 10.1016/j.chemgeo.2016.02.013
|
| [27] |
KARIMIAN N, JOHNSTON S G, BURTON E D, 2017. Acidity generation accompanying iron and sulfur transformations during drought simulation of freshwater re-flooded acid sulfate soils[J]. Geoderma, 285: 117-131.
doi: 10.1016/j.geoderma.2016.09.030
|
| [28] |
KARIMIAN N, JOHNSTON S G, BURTON E D, 2018. Iron and sulfur cycling in acid sulfate soil wetlands under dynamic redox conditions: A review[J]. Chemosphere, 197: 803-816.
doi: S0045-6535(18)30113-9
pmid: 29407844
|
| [29] |
KING G M, HOWES B L, DACEY J, 1985. Short-term endproducts of sulfate reduction in a salt marsh: Formation of acid volatile sulfides, elemental sulfur, and pyrite[J]. Geochimica et Cosmochimica Acta, 49(7): 1561-1566.
doi: 10.1016/0016-7037(85)90261-3
|
| [30] |
KRAAL P, BURTON E D, BUSH R T, 2013. Iron monosulfide accumulation and pyrite formation in eutrophic estuarine sediments[J]. Geochimica et Cosmochimica Acta, 122: 75-88.
doi: 10.1016/j.gca.2013.08.013
|
| [31] |
LICHTSCHLAG A, 2013. Intermediate sulfur oxidation state compounds in the euxinic surface sediments of the Dvurechenskii mud volcano (Black Sea)[J]. Geochimica et Cosmochimica Acta, 105: 130-145.
doi: 10.1016/j.gca.2012.11.025
|
| [32] |
LIN QI, WANG JIASHENG, FU SHAOYING, et al, 2015. Elemental sulfur in northern South China Sea sediments and its significance[J]. Science China Earth Sciences, 58(12): 2271-2278.
doi: 10.1007/s11430-015-5182-7
|
| [33] |
LIU JIARUI, PELLERIN A, ANTLER G, et al, 2020. Early diagenesis of iron and sulfur in Bornholm Basin sediments: The role of near-surface pyrite formation[J]. Geochimica et Cosmochimica Acta, 284: 43-60.
doi: 10.1016/j.gca.2020.06.003
|
| [34] |
LJUNG K, MALEY F, COOK A, et al, 2009. Acid sulfate soils and human health - A millennium ecosystem assessment[J]. Environment International, 35(8): 1234-1242.
doi: 10.1016/j.envint.2009.07.002
|
| [35] |
MACDONALD B C T, SMITH J, KEENE A F, et al, 2004. Impacts of runoff from sulfuric soils on sediment chemistry in an estuarine lake[J]. Science of the Total Environment, 329(1-3): 115-130.
pmid: 15262162
|
| [36] |
MELTON E D, SWANNER E D, BEHRENS S, et al, 2014. The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle[J]. Nature Reviews Microbiology, 12(12): 797 -808.
doi: 10.1038/nrmicro3347
pmid: 25329406
|
| [37] |
MORSE J W, THOMSON H, FINNERAN D W, 2007. Factors controlling sulfide geochemistry in sub-tropical estuarine and bay sediments[J]. Aquatic Geochemistry, 13(2): 143-156.
doi: 10.1007/s10498-007-9012-1
|
| [38] |
NÓBREGA G N, FERREIRA T O, ROMERO R E, et al, 2013. Iron and sulfur geochemistry in semi-arid mangrove soils (Ceará, Brazil) in relation to seasonal changes and shrimp farming effluents[J]. Environmental Monitoring and Assessment, 185(9): 7393-7407.
doi: 10.1007/s10661-013-3108-4
pmid: 23355026
|
| [39] |
OUESLATI W, ADDED A, ABDELJAOUED S, 2010. Vertical profiles of simultaneously extracted metals (SEM) and acid-volatile sulfide in a changed sedimentary environment: Ghar El Melh Lagoon, Tunisia[J]. Soil & Sediment Contamination: An International Journal, 19(6): 696-706.
|
| [40] |
RICKARD D, MORSE J W, 2005. Acid volatile sulfide (AVS)[J]. Marine Chemistry, 97(3-4): 141-197.
doi: 10.1016/j.marchem.2005.08.004
|
| [41] |
SCHOEPFER V A, BERNHARDT E S, BURGIN A J, 2014. Iron clad wetlands: Soil iron-sulfur buffering determines coastal wetland response to salt water incursion[J]. Biogeosciences, 119(12): 2209-2219.
|
| [42] |
SHENG YANQING, FU GUANGYI, CHEN FANZHONG, et al, 2011. Geochemical characteristics of inorganic sulfur in Shijing River, South China[J]. Journal of Environmental Monitoring, 13(4): 807-812.
doi: 10.1039/c0em00589d
pmid: 21267498
|
| [43] |
SMITH J, MELVILLE D M, 2004. Iron monosulfide formation and oxidation in drain-bottom sediments of an acid sulfate soil environment[J]. Applied Geochemistry, 19(11): 1837-1853.
doi: 10.1016/j.apgeochem.2004.04.004
|
| [44] |
WU ZIJUN, REN DEZHANG, ZHOU HUAIYANG, et al, 2016. Sulfate reduction and formation of iron sulfide minerals in nearshore sediments from Qi’ao Island, Pearl River Estuary, Southern China[J]. Quaternary International, 452(15): 137-147.
doi: 10.1016/j.quaint.2016.06.003
|
| [45] |
YÜCEL M, KONOVALOV S K, MOORE T S, et al, 2010. Sulfur speciation in the upper Black Sea sediments[J]. Chemical Geology, 269(3-4): 364-375.
doi: 10.1016/j.chemgeo.2009.10.010
|
| [46] |
ZHU MAOXU, LIU JUAN, YANG GUIPING, et al, 2012. Reactive iron and its buffering capacity towards dissolved sulfide in sediments of Jiaozhou Bay, China[J]. Marine environmental research, 80: 46-55.
doi: 10.1016/j.marenvres.2012.06.010
|
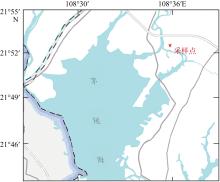
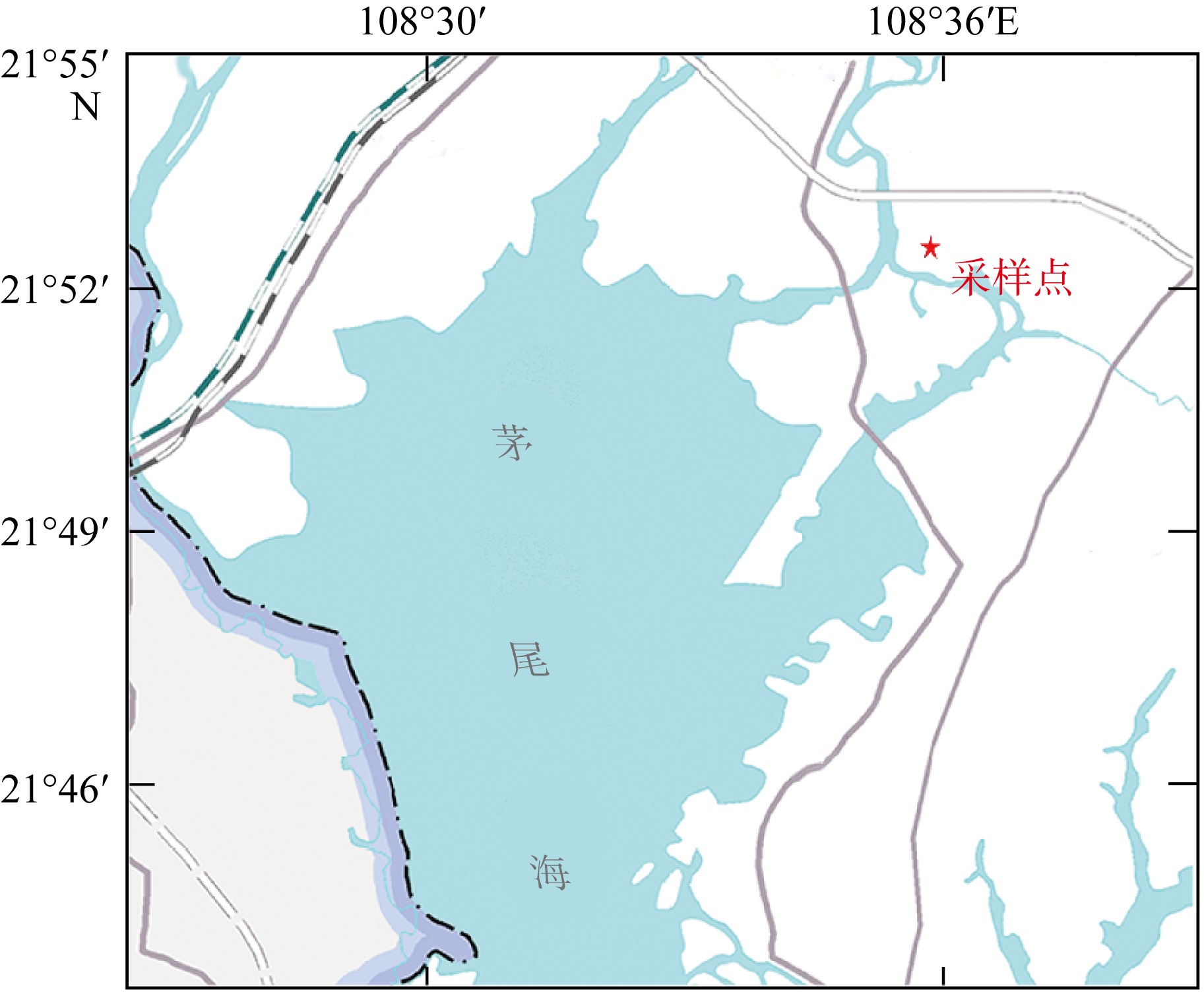
 ), 覃子东1, 王锋1,2, 蔡平雄1,2, 张生银3
), 覃子东1, 王锋1,2, 蔡平雄1,2, 张生银3
 ), QIN Zidong1, WANG Feng1,2, CAI Pingxiong1,2, ZHANG Shengyin3
), QIN Zidong1, WANG Feng1,2, CAI Pingxiong1,2, ZHANG Shengyin3