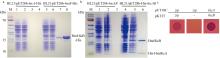
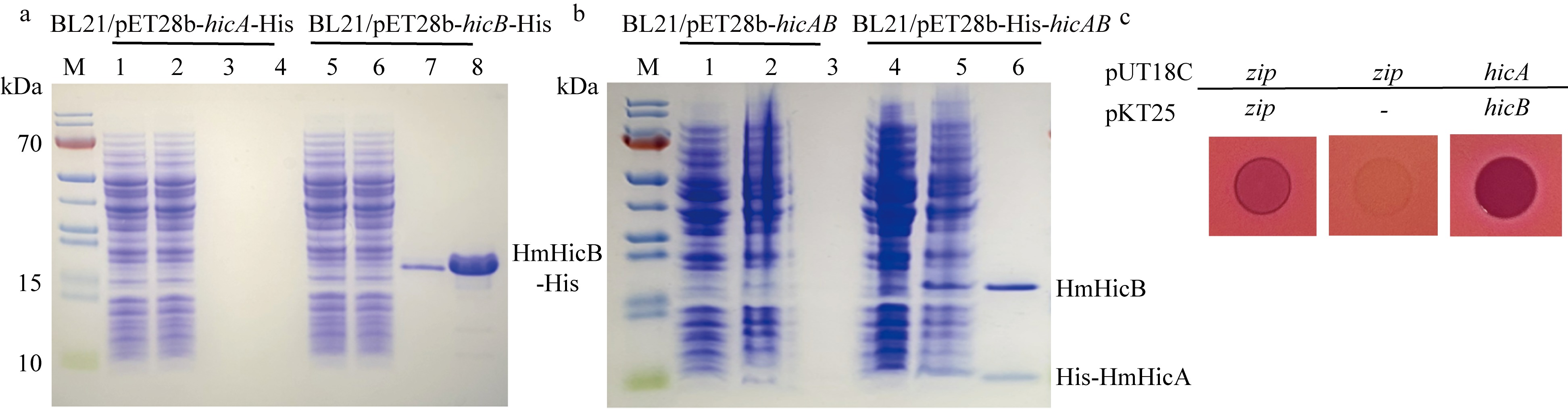
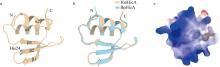
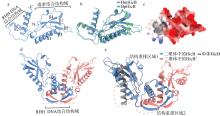
| [1] |
AAKRE C D, PHUNG T N, HUANG D, et al, 2013. A bacterial toxin inhibits DNA replication elongation through a direct interaction with the β sliding clamp[J]. Molecular Cell, 52(5): 617-628.
doi: 10.1016/j.molcel.2013.10.014
pmid: 24239291
|
| [2] |
BATTESTI A, BOUVERET E, 2012. The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli[J]. Methods, 58(4): 325-334.
doi: 10.1016/j.ymeth.2012.07.018
|
| [3] |
BECK I N, ARROWSMITH T J, GROBBELAAR M J, et al, 2024. Toxin release by conditional remodelling of ParDE1 from Mycobacterium tuberculosis leads to gyrase inhibition[J]. Nucleic Acids Research, 52(4): 1909-1929.
doi: 10.1093/nar/gkad1220
|
| [4] |
BIBI-TRIKI S, DE LA SIERRA-GALLAY I L, LAZAR N, et al, 2014. Functional and structural analysis of HicA3-HicB3, a novel toxin-antitoxin system of Yersinia pestis[J]. Journal of Bacteriology, 196(21): 3712-3723.
doi: 10.1128/JB.01932-14
|
| [5] |
BIKMETOV D, HALL A M J, LIVENSKYI A, et al, 2022. GNAT toxins evolve toward narrow tRNA target specificities[J]. Nucleic Acids Research, 50(10): 5807-5817.
doi: 10.1093/nar/gkac356
|
| [6] |
BUTT A, HIGMAN V A, WILLIAMS C, et al, 2014. The HicA toxin from Burkholderia pseudomallei has a role in persister cell formation[J]. Biochemical Journal, 459(2): 333-344.
doi: 10.1042/BJ20140073
|
| [7] |
CHEN RAN, ZHOU JIE, SUN RUNLIN, et al, 2020. Conserved conformational changes in the regulation of Mycobacterium tuberculosis MazEF-Mt1[J]. ACS Infectious Diseases, 6(7): 1783-1795.
doi: 10.1021/acsinfecdis.0c00048
|
| [8] |
DU XINLIN, VOLKOV O A, CZERWINSKI R M, et al, 2019. Structural basis and kinetic pathway of RBM39 recruitment to DCAF15 by a sulfonamide molecular glue E7820[J]. Structure, 27(11): 1625-1633. e3.
doi: S0969-2126(19)30346-6
pmid: 31693911
|
| [9] |
FRAIKIN N, GOORMAGHTIGH F, VAN MELDEREN L, 2020. Type Ⅱ toxin-antitoxin systems: evolution and revolutions[J]. Journal of Bacteriology, 202(7): e00763-19.
|
| [10] |
GARCIA-PINO A, DE GIETER S, TALAVERA A, et al, 2016. An intrinsically disordered entropic switch determines allostery in Phd-Doc regulation[J]. Nature Chemical Biology, 12(7): 490-496.
doi: 10.1038/nchembio.2078
|
| [11] |
GOULARD C, LANGRAND S, CARNIEL E, et al, 2010. The Yersinia pestis chromosome encodes active addiction toxins[J]. Journal of Bacteriology, 192(14): 3669-3677.
doi: 10.1128/JB.00336-10
|
| [12] |
GUAN JIAHAO, CHEN YONGKUI, GOH Y X, et al, 2024. TADB 3. 0: an updated database of bacterial toxin-antitoxin loci and associated mobile genetic elements[J]. Nucleic Acids Research, 52(D1): D784-D790.
doi: 10.1093/nar/gkad962
|
| [13] |
GUO YUNXUE, SUN CHENGLONG, LI YANGMEI, et al, 2019. Antitoxin HigA inhibits virulence gene mvfR expression in Pseudomonas aeruginosa[J]. Environmental Microbiology, 21(8): 2707-2723.
doi: 10.1111/emi.2019.21.issue-8
|
| [14] |
GUO YUNXUE, TANG KAIHAO, SIT B, et al, 2024. Control of lysogeny and antiphage defense by a prophage-encoded kinase-phosphatase module[J]. Nature Communications, 15(1): 7244.
doi: 10.1038/s41467-024-51617-x
pmid: 39174532
|
| [15] |
HAYES F, VAN MELDEREN L, 2011. Toxins-antitoxins: diversity, evolution and function[J]. Critical Reviews in Biochemistry and Molecular Biology, 46(5): 386-408.
doi: 10.3109/10409238.2011.600437
pmid: 21819231
|
| [16] |
HOLM L, 2022. Dali server: structural unification of protein families[J]. Nucleic Acids Research, 50(W1): W210-W215.
doi: 10.1093/nar/gkac387
pmid: 35610055
|
| [17] |
JØRGENSEN M G, PANDEY D P, JASKOLSKA M, et al, 2009. HicA of Escherichia coli defines a novel family of translation-independent mRNA interferases in bacteria and archaea[J]. Journal of Bacteriology, 191(4): 1191-1199.
doi: 10.1128/JB.01013-08
|
| [18] |
JURĖNAS D, FRAIKIN N, GOORMAGHTIGH F, et al, 2022. Biology and evolution of bacterial toxin-antitoxin systems[J]. Nature Reviews Microbiology, 20(6): 335-350.
doi: 10.1038/s41579-021-00661-1
pmid: 34975154
|
| [19] |
KIM D H, KANG S M, PARK S J, et al, 2018. Functional insights into the Streptococcus pneumoniae HicBA toxin-antitoxin system based on a structural study[J]. Nucleic Acids Research, 46(12): 6371-6386.
doi: 10.1093/nar/gky469
|
| [20] |
LIN JIANZHONG, GUO YUNXUE, YAO JIANYUN, et al, 2023. Applications of toxin-antitoxin systems in synthetic biology[J]. Engineering Microbiology, 3(2): 100069.
doi: 10.1016/j.engmic.2023.100069
|
| [21] |
LIU ZIYAO, TANG KAIHAO, ZHOU YIQING, et al, 2024. Active prophages in coral-associated Halomonas capable of lateral transduction[J]. The ISME Journal, 18(1): wrae085.
doi: 10.1093/ismejo/wrae085
|
| [22] |
MAKAROVA K S, GRISHIN N V, KOONIN E V, 2006. The HicAB cassette, a putative novel, RNA-targeting toxin-antitoxin system in archaea and bacteria[J]. Bioinformatics, 22(21): 2581-2584.
pmid: 16895922
|
| [23] |
MANAV M C, TURNBULL K J, JURĖNAS D, et al, 2019. The E. coli HicB antitoxin contains a structurally stable helix-turn-helix DNA binding domain[J]. Structure, 27(11): 1675-1685. e3.
doi: 10.1016/j.str.2019.08.008
|
| [24] |
MASUDA H, TAN QIAN, AWANO N, et al, 2012. A novel membrane-bound toxin for cell division, CptA (YgfX), inhibits polymerization of cytoskeleton proteins, FtsZ and MreB, in Escherichia coli[J]. FEMS Microbiology Letters, 328(2): 174-181.
doi: 10.1111/fml.2012.328.issue-2
|
| [25] |
MENG E C, GODDARD T D, PETTERSEN E F, et al, 2023. UCSF ChimeraX: Tools for structure building and analysis[J]. Protein Science, 32(11): e4792.
doi: 10.1002/pro.4792
pmid: 37774136
|
| [26] |
MHLANGA-MUTANGADURA T, MORLIN G, SMITH A L, et al, 1998. Evolution of the major Pilus gene cluster of Haemophilus influenzae[J]. Journal of Bacteriology, 180(17): 4693-4703.
doi: 10.1128/JB.180.17.4693-4703.1998
|
| [27] |
MILLMAN A, BERNHEIM A, STOKAR-AVIHAIL A, et al, 2020. Bacterial retrons function in anti-phage defense[J]. Cell, 183(6): 1551-1561.
doi: 10.1016/j.cell.2020.09.065
pmid: 33157039
|
| [28] |
NI SONGWEI, LI BAIYUAN, TANG KAIHAO, et al, 2021. Conjugative plasmid-encoded toxin-antitoxin system PrpT/PrpA directly controls plasmid copy number[J]. Proceedings of the National Academy of Sciences of the United States of America, 118(4): e2011577118.
|
| [29] |
ROCKER A, PESCHKE M, KITTILÄ T, et al, 2018. The ng_ζ1 toxin of the gonococcal Epsilon/Zeta toxin/antitoxin system drains precursors for cell wall synthesis[J]. Nature Communications, 9(1): 1686.
doi: 10.1038/s41467-018-03652-8
|
| [30] |
SOO V W C, WOOD T K, 2013. Antitoxin MqsA represses curli formation through the master biofilm regulator CsgD[J]. Scientific Reports, 3: 3186.
doi: 10.1038/srep03186
pmid: 24212724
|
| [31] |
TANG KAIHAO, WANG WEIQUAN, SUN YAMIN, et al, 2021. Prophage Tracer: precisely tracing prophages in prokaryotic genomes using overlapping split-read alignment[J]. Nucleic Acids Research, 49(22): e128.
doi: 10.1093/nar/gkab824
pmid: 34551431
|
| [32] |
VARADI M, BERTONI D, MAGANA P, et al, 2024. AlphaFold protein structure database in 2024: providing structure coverage for over 214 million protein sequences[J]. Nucleic Acids Research, 52(D1): D368-D375.
doi: 10.1093/nar/gkad1011
|
| [33] |
WANG XIAOXUE, KIM Y, HONG S H, et al, 2011. Antitoxin MqsA helps mediate the bacterial general stress response[J]. Nature Chemical Biology, 7(6): 359-366.
doi: 10.1038/nchembio.560
pmid: 21516113
|
| [34] |
WINTER A J, WILLIAMS C, ISUPOV M N, et al, 2018. The molecular basis of protein toxin HicA-dependent binding of the protein antitoxin HicB to DNA[J]. Journal of Biological Chemistry, 293(50): 19429-19440.
doi: 10.1074/jbc.RA118.005173
pmid: 30337369
|
| [35] |
XUE LU, YUE JIAN, KE JIYUAN, et al, 2020. Distinct oligomeric structures of the YoeB-YefM complex provide insights into the conditional cooperativity of type Ⅱ toxin-antitoxin system[J]. Nucleic Acids Research, 48(18): 10527-10541.
doi: 10.1093/nar/gkaa706
|
| [36] |
YAO JIANYUN, ZHEN XIANGKAI, TANG KAIHAO, et al, 2020. Novel polyadenylylation-dependent neutralization mechanism of the HEPN/MNT toxin/antitoxin system[J]. Nucleic Acids Research, 48(19): 11054-11067.
doi: 10.1093/nar/gkaa855
pmid: 33045733
|
| [37] |
ZIANNI M, TESSANNE K, MERIGHI M, et al, 2006. Identification of the DNA bases of a DNase I footprint by the use of dye primer sequencing on an automated capillary DNA analysis instrument[J]. Journal of Biomolecular Techniques, 17(2): 103-113.
pmid: 16741237
|
 ), LIU Ziyao2, WANG Xiaoxue2, CHEN Ran2(
), LIU Ziyao2, WANG Xiaoxue2, CHEN Ran2( )
)