热带海洋学报 ›› 2024, Vol. 43 ›› Issue (1): 94-106.doi: 10.11978/2023040CSTR: 32234.14.2023040
牙鲆(Paralichthys olivaceus)抗缪勒氏管激素Ⅱ型受体基因(amhr2)表达特征分析及功能初探
李泽1,2,3( ), 王丽娟1,2(
), 王丽娟1,2( ), 邹聪聪1,2,3, 舒畅1,2,3, 吴志昊1,2, 邹玉霞1,2, 尤锋1,2(
), 邹聪聪1,2,3, 舒畅1,2,3, 吴志昊1,2, 邹玉霞1,2, 尤锋1,2( )
)
- 1.中国科学院实验海洋生物学重点实验室, 海洋大科学中心(中国科学院海洋研究所), 山东省实验海洋生物学重点实验室, 山东 青岛 266071
2.青岛海洋科学与技术试点国家实验室, 海洋生物学与生物技术实验室, 山东 青岛 266237
3.中国科学院大学, 北京 100049
-
收稿日期:2023-03-24修回日期:2023-04-21出版日期:2024-01-10发布日期:2024-01-19 -
作者简介:李泽 (1998—), 男, 山东青岛人, 硕士研究生, 主要从事鲆鲽鱼类发育生物学研究。email: lize20@qdio.ac.cn
-
基金资助:国家重点研发计划(2022YFD2400402); 国家重点研发计划(2018YFD0900202); 山东省自然科学基金(ZR2022MC026); 青岛海洋科学与技术试点国家实验室海洋生物学与生物技术功能实验室青年科学基金项目(YQ2018NO01)
Expression characteristics and function of Anti-Müllerian hormone receptor Ⅱ gene (amhr2) in Paralichthys olivaceus
LI Ze1,2,3( ), WANG Lijuan1,2(
), WANG Lijuan1,2( ), ZOU Congcong1,2,3, SHU Chang1,2,3, WU Zhihao1,2, ZOU Yuxia1,2, YOU Feng1,2(
), ZOU Congcong1,2,3, SHU Chang1,2,3, WU Zhihao1,2, ZOU Yuxia1,2, YOU Feng1,2( )
)
- 1. Chinese Academy of Sciences and Shandong Province Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science (Institute of Oceanology, Chinese Academy of Sciences), Qingdao 266071, China
2. Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
3. University of Chinese Academy of Sciences, Beijing 100049, China
-
Received:2023-03-24Revised:2023-04-21Online:2024-01-10Published:2024-01-19 -
Supported by:National Key R&D Program of China(2022YFD2400402); National Key R&D Program of China(2018YFD0900202); Shandong Natural Science Foundation(ZR2022MC026); Youth Research Fund of Marine Biology and Biotechnology Laboratory, Pilot National Laboratory for Marine Science and Technology (Qingdao)(YQ2018NO01)
摘要:
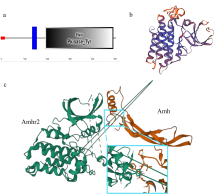
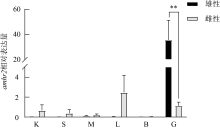
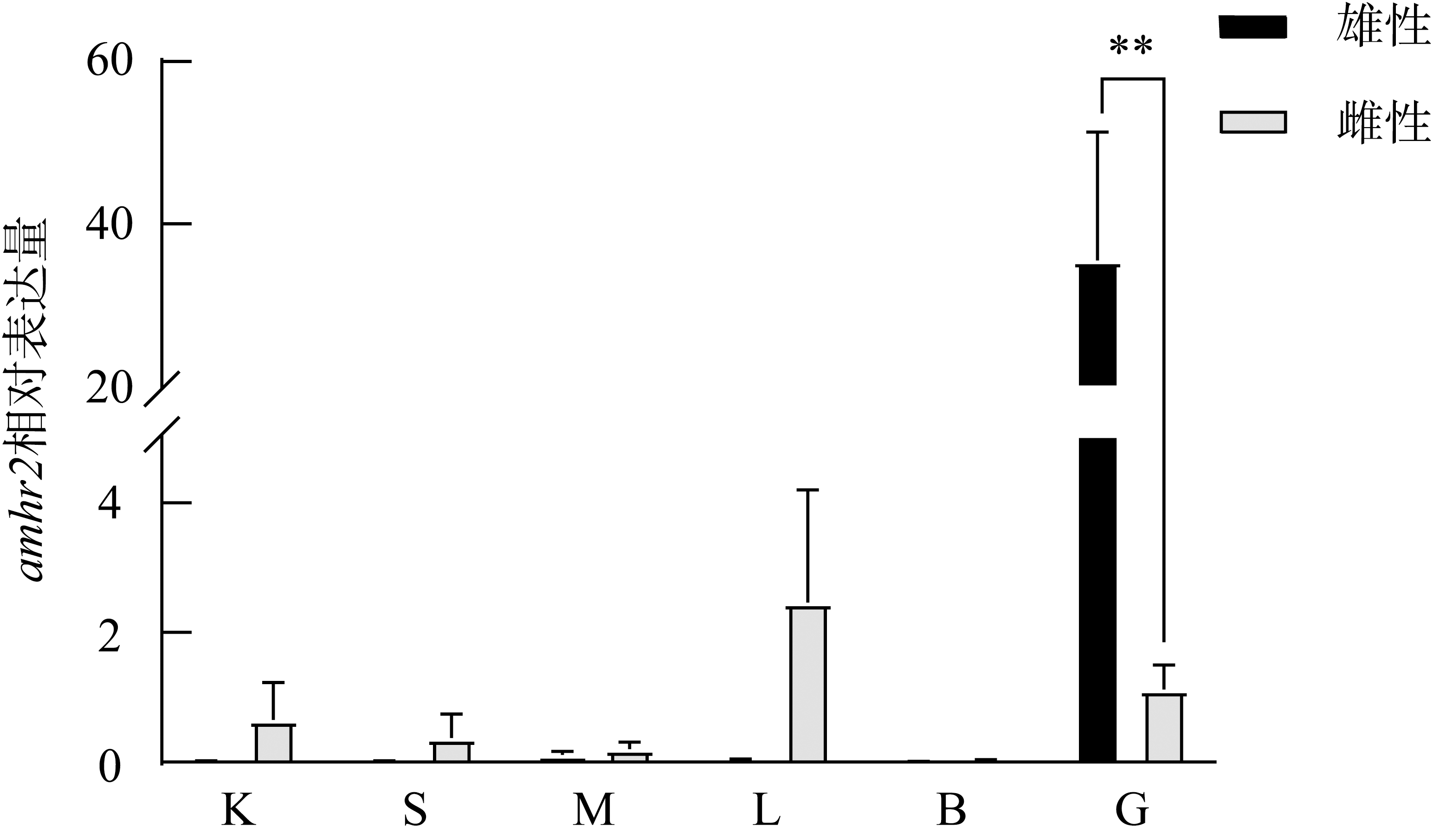
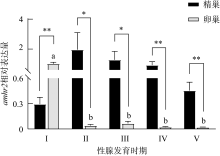
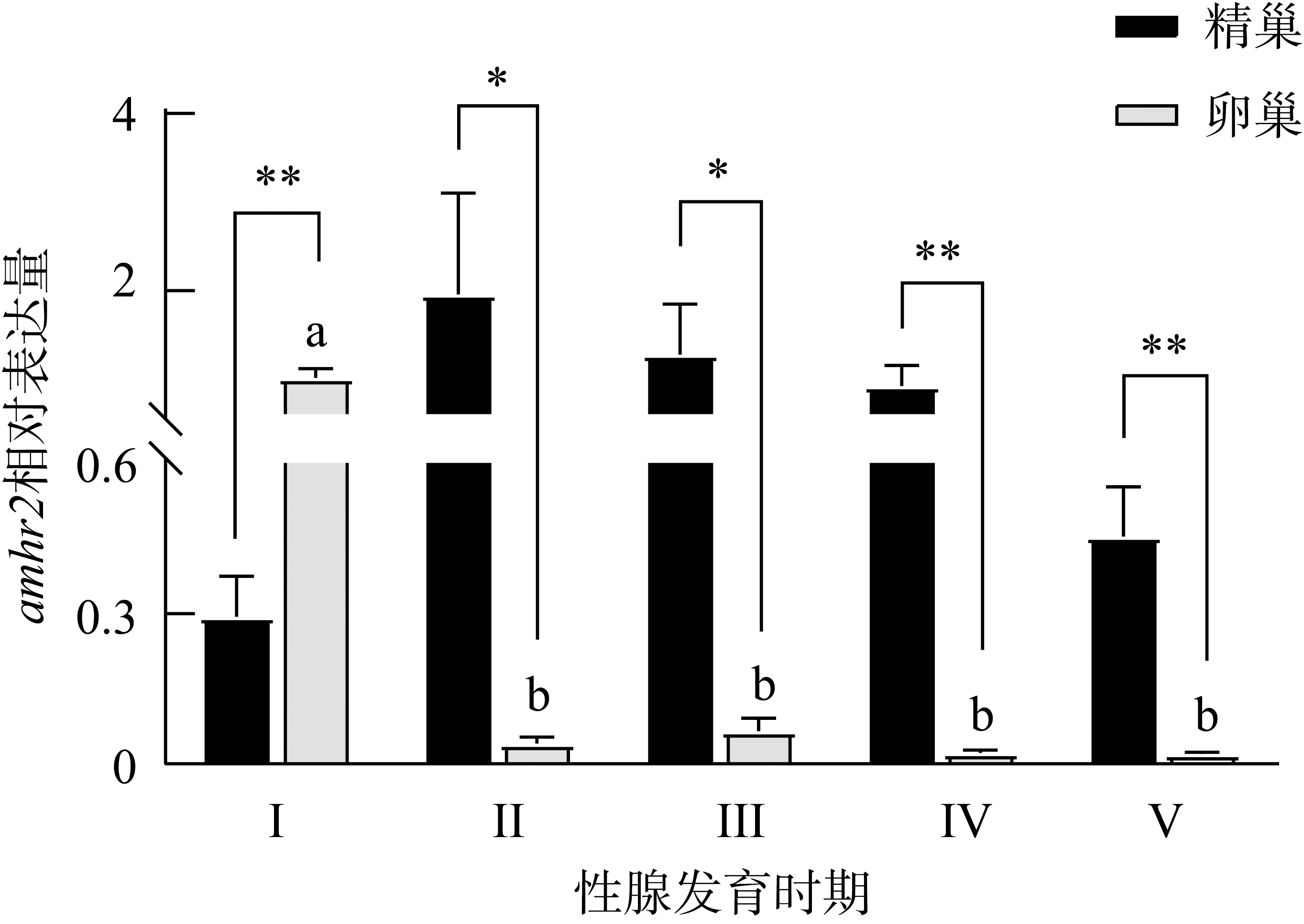
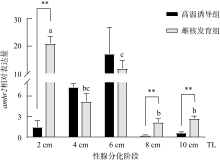
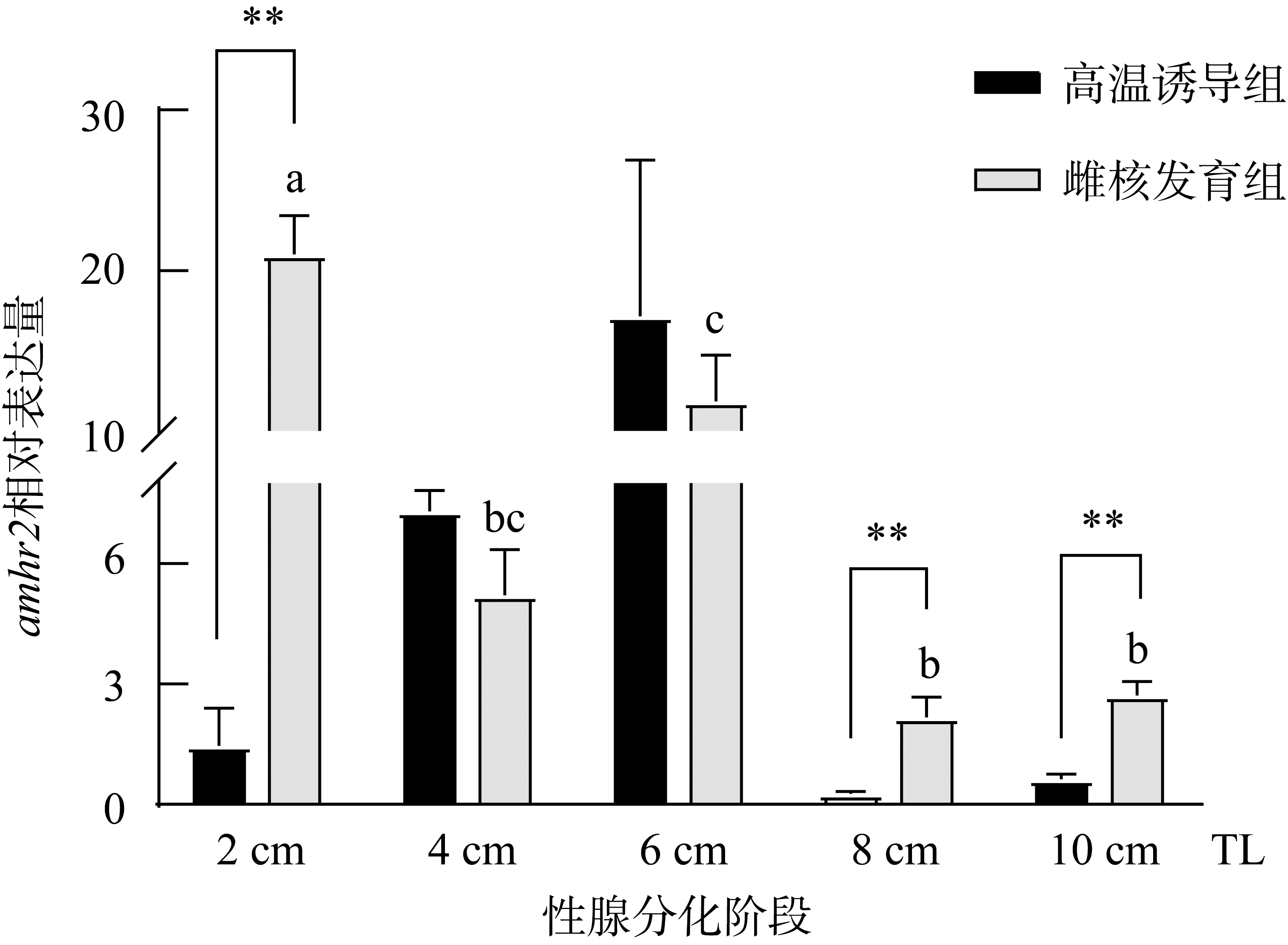
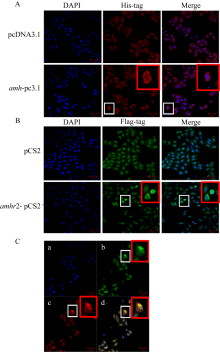
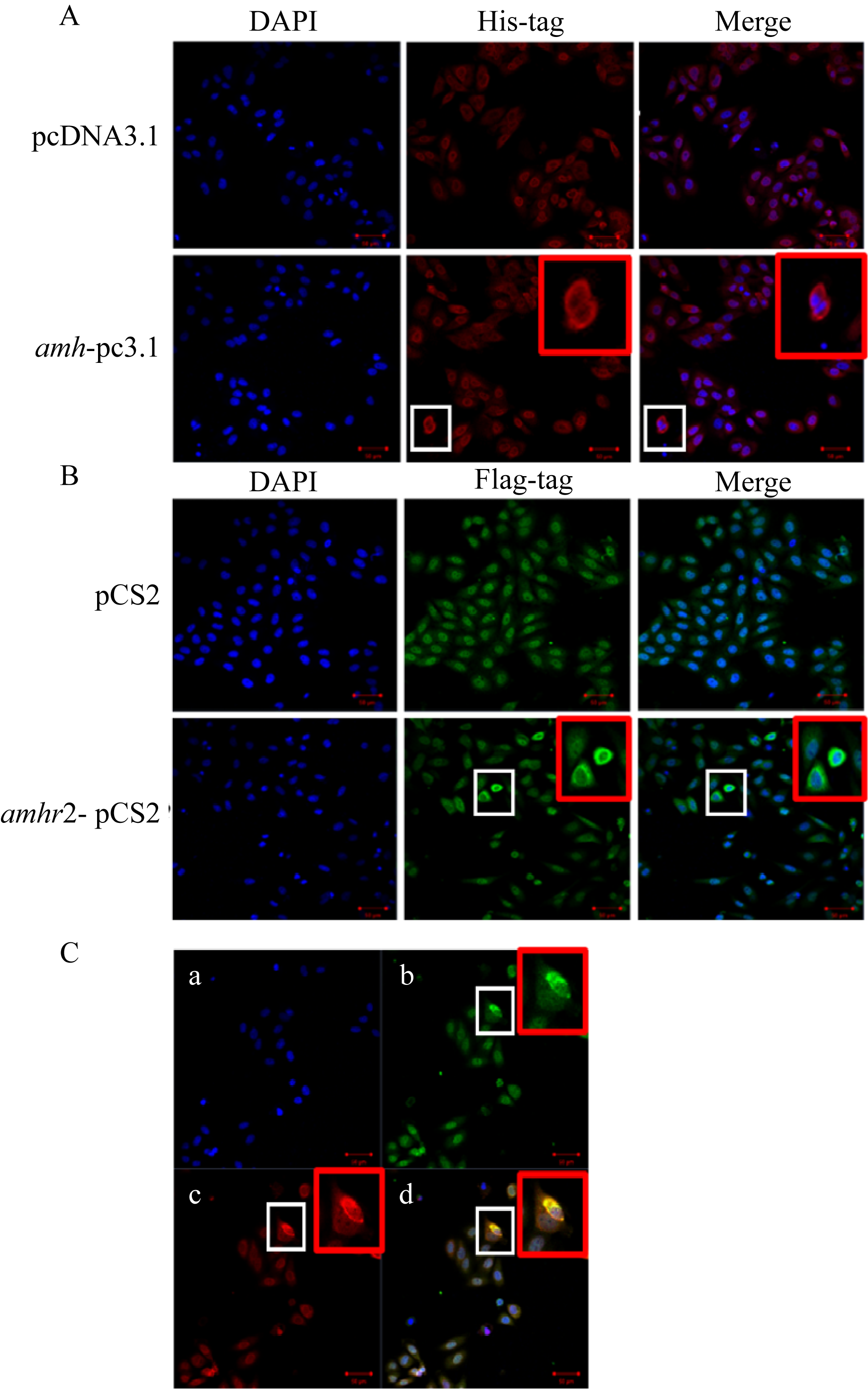
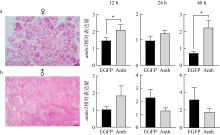
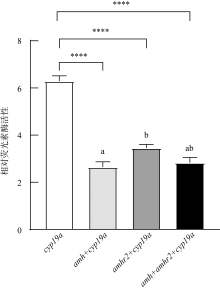
抗缪勒氏管激素Ⅱ型受体(anti-Müllerian hormone receptor Ⅱ, Amhr2)是抗缪勒氏管激素(anti-Müllerian hormone, Amh)的特异受体。amhr2基因在鱼类性腺分化和发育中发挥重要作用, 更决定了有的鱼种的性别, 然而, 相关功能研究较为有限。本研究旨在明晰amhr2在我国重要海水养殖鱼类—牙鲆(Paralichthys olivaceus)性腺分化和发育过程中的表达特征, 并初步探究其功能。首先, 克隆了牙鲆amhr2-CDS序列, 共1536bp, 编码511个氨基酸。系统发育分析显示牙鲆Amhr2近C端较为保守, 与其他硬骨鱼类聚为一枝, 并与上游sp1以及下游的prr13和PCBP2在基因组中共定位。蛋白结构分析显示, 牙鲆Amhr2包含信号肽、跨膜结构域和保守的酪氨酸蛋白激酶结构域。进而, 利用实时荧光定量PCR (qPCR)分析表明, 牙鲆amhr2主要表达于性腺, 且在精巢中的表达极显著高于卵巢(P< 0.01), 其在I-V期精巢中持续高表达, 而在卵巢中仅在I期高表达, 后显著下降(P< 0.05)。性腺分化期基因的表达, 是对雌核发育组(对照组, 20 ± 0.5℃, 100%雌性)与雌核发育高温诱导组(HT组, 28 ± 0.5℃, 100%雄性)鱼苗进行检测的, amhr2在对照组全长(total length, TL)2cm的实验鱼性腺中表达最高, 后逐渐下降; 而HT组实验鱼性腺中的表达呈现先上升后下降的趋势, 并在精巢开始分化的6cm TL时表达最高。利用Hela细胞进行亚细胞定位分析发现, Amhr2与其配体Amh均定位于细胞质。同时, 通过原核表达重组牙鲆Amh, 并用其孵育离体性腺组织, qPCR分析显示精巢中amhr2的表达没有显著变化, 但卵巢中表达显著上升(P< 0.05)。进一步通过双荧光素酶报告分析表明, Amh和Amhr2共转染能够显著抑制雌激素合成的关键芳香化酶基因cyp19a的表达(P< 0.01)。综上所述, 牙鲆amhr2主要表达于精巢, 但在不同性腺发育时期表达不同, 且其可能与Amh共同影响cyp19a的转录, 对雄性表型形成的启动和性腺发育起作用。
引用本文
李泽, 王丽娟, 邹聪聪, 舒畅, 吴志昊, 邹玉霞, 尤锋. 牙鲆(Paralichthys olivaceus)抗缪勒氏管激素Ⅱ型受体基因(amhr2)表达特征分析及功能初探[J]. 热带海洋学报, 2024, 43(1): 94-106.
LI Ze, WANG Lijuan, ZOU Congcong, SHU Chang, WU Zhihao, ZOU Yuxia, YOU Feng. Expression characteristics and function of Anti-Müllerian hormone receptor Ⅱ gene (amhr2) in Paralichthys olivaceus[J]. Journal of Tropical Oceanography, 2024, 43(1): 94-106.
表1
本研究所用引物"
| 引物 | 序列 | NCBI序列号及参考文献 |
|---|---|---|
| amhr2-CDS-F | ATTCCCAGGACCCTCCCTGTT | XM_020096590.1 |
| amhr2-CDS-R | TCTGCAAAGAGAACAGTGCAAC | |
| amh-CDS-F | ATGCCGGTGGTGAACGTCTT | XM_020080898.1 |
| amh-CDS-R | GCGGCATCCACACTCCTTTG | |
| amhr2-qPCR-F | CGTCGTGTTCCTGCCAAAC | XM_020096590.1 |
| amhr2-qPCR-R | GGCAGCTCATACACCTCCTT | |
| actin- qPCR-F | GGAATCCACGAGACCACCTACA | EU090804 Hu et al, |
| actin- qPCR-R | CTGCTTGCTGATCCACATCTGC | |
| ef- qPCR-F | AGCCAGAAGCGTTTTGAGGAG | XM_020104638.1 Zhong et al, |
| ef- qPCR-R | AGATGGGGACGAAAGCAACAC | |
| amh-pc3.1-EcoRV-F | CACACTGGACTAGTGGATCCGCCACCATGCCGGTGGTGAACGTC | XM_020080898.1 |
| amh-pc3.1-EcoRV-R | AGCTTGGTACCGAGCTCGGATCCGCGGCATCCACACTCCT | |
| amhr2-pc3.1-BamHI-F | CACACTGGACTAGTGGATCCGCCACCATGATGCTGCAGTGGTGGC | XM_020096590.1 |
| amhr2-pc3.1-BamHI-R | AGCTTGGTACCGAGCTCGGATCCTGAGCTGCTTTCAGAGACATAAGACTG | |
| amhr2-pCS2-BamHI-F | ACGACGACGATAAGGGATCCGCCACCATGATGCTGCAGTGGTGGC | XM_020096590.1 |
| amhr2-pCS2-BamHI-R | AATTCGAATCGATGGGATCCTGAGCTGCTTTCAGAGACATAAGACTG | |
| amh-pET-EcoRV-F | AAGGCCATGGCTGATATGCCGGTGGTGAACGTCTT | XM_020080898.1 |
| amh-pET-EcoRV-R | GAATTCGGATCCGATGCGGCATCCACACTCCTTTG |
| [1] |
范兆飞, 2017. 牙鲆cyp19a及其转录因子表观修饰和调控研究[D]. 青岛: 中国科学院大学: 中国科学院海洋研究所: 1-137.
|
|
|
|
| [2] |
高莹莹, 胡鹏, 刘新富, 等, 2019. 暗纹东方鲀抗苗勒氏管激素Ⅱ型受体基因的克隆、生物信息学及表达分析[J]. 海洋渔业, 41(5): 555-566.
|
|
|
|
| [3] |
韩玉龙, 2019. amh基因在斜带石斑鱼性别分化中的作用机制研究[D]. 广州: 中山大学: 1-130.
|
|
|
|
| [4] |
孙鹏, 尤锋, 张立敬, 等, 2009. 牙鲆性腺分化的组织学研究[J]. 海洋科学, 33(3): 53-58.
|
|
|
|
| [5] |
王妹, 邓思平, 陈华谱, 等, 2018. 金钱鱼Amhr2基因的克隆及表达分析[J]. 广东海洋大学学报, 38(3): 17-24.
|
|
|
|
| [6] |
赵九娥, 2015. Amhy/Amh及其受体AmhrⅡ对尼罗罗非鱼雄性性别的决定作用[D]. 重庆: 西南大学: 1-80.
|
|
|
|
| [7] |
doi: 10.1038/ncomms10055 pmid: 26753790 |
| [8] |
doi: 10.1007/s11033-021-06606-4 |
| [9] |
|
| [10] |
doi: 10.1016/j.aquaculture.2018.03.008 |
| [11] |
doi: 10.3389/fendo.2019.00210 |
| [12] |
|
| [13] |
doi: 10.3389/fgene.2022.1007548 |
| [14] |
doi: 10.1073/pnas.1018392109 pmid: 22323585 |
| [15] |
|
| [16] |
doi: 10.1371/journal.pone.0108582 |
| [17] |
|
| [18] |
doi: 10.1371/journal.pgen.1002798 |
| [19] |
doi: 10.1002/dvdy.v236:1 |
| [20] |
|
| [21] |
doi: 10.1371/journal.pgen.1005678 |
| [22] |
doi: 10.1677/jme.1.01853 |
| [23] |
|
| [24] |
doi: 10.1098/rstb.2020.0091 |
| [25] |
doi: 10.1016/j.ygcen.2015.09.025 |
| [26] |
doi: 10.1016/j.modgep.2005.02.008 |
| [27] |
doi: 10.1371/journal.pone.0081551 |
| [28] |
|
| [29] |
doi: 10.1007/s11802-022-4898-1 |
| [30] |
doi: 10.1016/j.ijbiomac.2022.06.098 |
| [31] |
doi: 10.1007/s00427-015-0495-2 |
| [32] |
doi: 10.1016/j.aquaculture.2022.738984 |
| [33] |
doi: 10.1016/j.gene.2020.144906 |
| [34] |
|
| [35] |
doi: 10.3390/ijms24032480 |
| [36] |
doi: 10.1016/j.cub.2012.05.045 |
| [37] |
doi: 10.1016/j.bbrc.2004.07.162 |
| [38] |
doi: 10.1007/BF02863039 |
| [39] |
doi: 10.3390/fishes7030129 |
| [40] |
|
| [41] |
|
| [42] |
doi: 10.1007/s10126-007-9064-7 |
| [1] | 饶义勇, 赵美榕, 旷泽行, 黄洪辉, 谭萼辉. 浮筏式牡蛎养殖对大型底栖动物群落功能结构的影响——以大鹏澳为例*[J]. 热带海洋学报, 2024, 43(5): 69-83. |
| [2] | 袁翔城, 梁宇娴, 宋严, 俞晓磊, 黄晖, 周伟华. CO2升高对风信子鹿角珊瑚(Acropora hyacinthus)钙化速率和基因表达的影响*[J]. 热带海洋学报, 2024, 43(3): 40-48. |
| [3] | 贾男, 周天成, 胡思敏, 张琛, 黄晖, 刘胜. 南沙群岛海域珊瑚礁区三种寄居蟹的摄食差异比较[J]. 热带海洋学报, 2024, 43(3): 109-121. |
| [4] | 孙婷婷, 郝雯瑾, 徐鹏臻, 叶丽靖, 董志军. 海水酸化对海月水母螅状体共附生微生物的影响[J]. 热带海洋学报, 2023, 42(6): 111-119. |
| [5] | 许瀚之, 张华, 熊盼盼, 何毛贤. 马氏珠母贝异速生长个体对免疫刺激的差异响应[J]. 热带海洋学报, 2022, 41(5): 180-188. |
| [6] | 潘肖兰, 刘惠茹, 许濛, 许瀚之, 张华, 何毛贤. 马氏珠母贝PfAQP4免疫功能初探[J]. 热带海洋学报, 2021, 40(2): 83-89. |
| [7] | 倪嘉豪, 朱晓静, 季益平, 周彬, 王亚军, 徐善良, 王丹丽. 养殖密度对银鲳幼鱼生长、代谢酶活力及其相关基因表达的影响[J]. 热带海洋学报, 2020, 39(2): 54-64. |
| [8] | 陆友云, 薛明, 李志桦, 温崇庆. 海洋蛭弧菌DA5全基因组测序及序列分析[J]. 热带海洋学报, 2018, 37(6): 112-119. |
| [9] | 王富轩, 肖述, 向志明, 喻子牛. 香港牡蛎 (Crassostrea hongkongensis) Commd1基因的分子克隆及其在盐度胁迫下的表达分析[J]. 热带海洋学报, 2017, 36(1): 48-55. |
| [10] | 沈城, 刘楚吾, 刘丽. 温度胁迫及恢复初期稀杯盔形珊瑚共生虫黄藻Hsp70、Hsp90、psaA、psbA基因表达分析[J]. 热带海洋学报, 2016, 35(3): 72-78. |
| [11] | 韩婷婷, 齐占会, 吴风霞, 廖秀丽, 马胜伟, 付贵权, 黄洪辉. 大亚湾不同海洋功能区表层海水无机碳体系的比较研究*[J]. 热带海洋学报, 2016, 35(2): 57-65. |
| [12] | 黄晖, 许昌有, 袁涛. 造礁石珊瑚白化相关功能基因的研究进展[J]. 热带海洋学报, 2013, 32(4): 43-50. |
| [13] | 张立娟,何毛贤. 生长激素与胰岛素样生长因子 - Ⅰ对马氏珠母贝壳生长形成相关基因表达的影响[J]. 热带海洋学报, 2012, 31(2): 96-101. |
|
||