

Journal of Tropical Oceanography >
Enantioselective production of (R)-1-phenylethanol by a novel marine microbial carboxylesterase
Received date: 2016-08-31
Request revised date: 2016-09-22
Online published: 2017-06-01
Supported by
Strategic Priority Research Program of the Chinese Academy of Sciences (XDA11030404)
Guangzhou Science and Technology Plan Projects (201510010012)
National Natural Science Foundation of China (21302199)
Copyright
Deep-sea microbial esterase BSE00077 can efficiently resolve (±)-1-phenylethyl acetate and generate (R)-1- phenylethanol. The optimal working conditions for the enzymatic resolution of (±)-1-phenylethyl acetate by marine microbial esterase BSE00077 were found to be: 50mmol/L (±)-1-phenylethyl acetate, 10% n-heptane (v/v), pH 7.0, 30°C for 2h. After process optimization, desired optically pure product (R)-1-phenylethanol was obtained with an enantiomeric excess of over 99% and a conversion of over 30%. Esterase BSE00077 can also enzymatically resolve some other racemic 1-phenylethyl esters with different chain lengths. We found that the chain lengths could greatly affect the enantiomeric excess of desired product. Esterase BSE00077 is a novel marine microbial esterase with great potential in the asymmetric synthesis of (R)-1-phenylethanol as well as of other chiral chemicals in industry.
HUANG Jinlong , ZHANG Yun , SUN Aijun , HU Yunfeng . Enantioselective production of (R)-1-phenylethanol by a novel marine microbial carboxylesterase[J]. Journal of Tropical Oceanography, 2017 , 36(3) : 55 -60 . DOI: 10.11978/2016081
Fig. 1 Enzymatic kinetic resolution of (±)-1-phenylethyl acetate by esterase BSE00077图1 酯酶BSE00077拆分乙酸苏合香酯 |
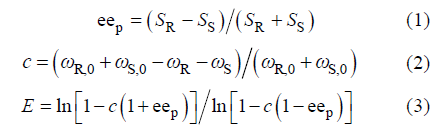
Tab. 1 Effects of temperature and pH on kinetic resolution by esterase BSE00077表1 温度和pH对酯酶BSE00077拆分的影响 |
| 温度/℃ | 产物对映体过量值/% | 底物转化率/% | pH | 产物对映体过量值/% | 底物转化率/% |
|---|---|---|---|---|---|
| 25 | 96.17 ± 0.47 | 37.12 ± 1.32 | 6 | 99.50 ± 0 | 9.20 ± 1.72 |
| 30 | 96.21 ± 0.88 | 42.51 ± 1.45 | 7 | 93.27 ± 1.08 | 41.64 ± 2.44 |
| 35 | 95.53 ± 0.35 | 44.54 ± 1.74 | 8 | 91.94 ± 0.64 | 45.32 ± 1.69 |
| 40 | 95.25 ± 0.95 | 37.28 ± 0.77 | 9 | 91.23 ± 0.52 | 47.10 ± 3.07 |
| 45 | 85.30 ± 0.81 | 9.96 ± 1.31 |
Tab. 2 Effects of organic solvents on kinetic resolution by esterase BSE00077表2 有机溶剂对酯酶BSE00077拆分的影响 |
| 有机溶剂 | lg P* | 产物对映体过量值/% | 底物转化率/% | 对映体比值E |
|---|---|---|---|---|
| 对照 | 94.35 ± 1.05 | 27.90 ± 0.79 | 49.38 ± 4.27 | |
| 二甲基亚砜 | -1.35 | 93.75 ± 0.67 | 31.76 ± 1.44 | 47.97 ± 4.38 |
| 甲醇 | -0.72 | 93.63 ± 0.45 | 7.33 ± 1.38 | 32.80 ± 1.95 |
| 甲苯 | 2.68 | >99.00 | 5.04 ± 0.69 | 209.65 ± 1.56 |
| 二甲苯 | 3.14 | 94.14 ± 0.88 | 14.37 ± 1.05 | 39.24 ± 3.68 |
| 正己烷 | 3.94 | 94.49 ± 0.95 | 31.73 ± 3.41 | 55.36 ± 6.25 |
| 正庚烷 | 4.47 | 94.81 ± 0.68 | 31.72 ± 2.44 | 58.53 ± 5.12 |
| 正辛烷 | 5.01 | 94.23 ± 0.79 | 32.03 ± 1.75 | 52.77 ± 5.76 |
注: *: 有机化合物脂水分配系数, 数值参考自www.chemspider.comr |
Fig. 2 Effects of substrate concentration on kinetic resolution图2 底物浓度对酯酶BSE00077拆分的影响 |
Tab. 3 Time proceeding of kinetic resolution of (R, S)-1- phenylethanol catalyzed by esterase BSE00077表3 酯酶BSE00077拆分(R, S)-1-苯乙醇的反应时间进程 |
| 时间/h | 产物对映体过量值/% | 底物转化率/% |
|---|---|---|
| 0.5 | 99.19 ± 0.14 | 23.79 ± 1.03 |
| 1 | 99.12 ± 0.12 | 25.68 ± 0.92 |
| 2 | 99.03 ± 0.09 | 30.71 ± 0.67 |
| 3 | 98.14 ± 0.48 | 36.22 ± 2.31 |
| 4 | 97.00 ± 0.47 | 40.60 ± 1.94 |
| 5 | 96.45 ± 1.01 | 42.33 ± 2.04 |
| 6 | 94.48 ± 0.89 | 43.57 ± 1.87 |
| 7 | 94.53 ± 0.36 | 43.53 ± 1.44 |
| 8 | 94.43 ± 0.42 | 43.61 ± 1.37 |
Tab. 4 Enzymatic kinetic resolution of 1-phenylethyl esters with different chain lengths by esterase BSE00077表4 酯酶BSE00077拆分不同酰基链长的1-苯乙醇酯 |
| 底物 | 反应 时间/h | 起始反应速率/(mmol·L-1·h-1) | 产物对映体 过量值/% | 底物转化率/% |
|---|---|---|---|---|
| 乙酸苏合香酯 | 0.5 | 23.79 | 99.19 ± 0.14 | 23.79 ± 1.03 |
| 丙酸苏合香酯 | 0.5 | 23.84 | 90.22 ± 0.51 | 23.84 ± 1.55 |
| 丁酸苏合香酯 | 0.5 | 10.40 | 91.67 ± 0.73 | 10.40 ± 0.28 |
Tab. 5 Comparison of resolution of 1-phenylethanol by different esterase/lipases表5 比较不同来源的酯酶和脂肪酶拆分1-苯乙醇 |
| 酶(来源) | 反应方式 | R/S- 苯乙醇 | 对映体过量值/% | 参考文献 |
|---|---|---|---|---|
| 脂肪酶Novozym 435(商业酶) | 转酯化 | S | 87.5 | (严祥辉 等, 2009) |
| C. antarctica 脂肪酶(商业酶) | 水解 | S | 99.5 | (Fan et al, 2011) |
| C. lipolytica 脂肪酶(商业酶) | 转酯化 | S | 99.5 | (范卫东 等, 2012) |
| 酯酶BSE00077 (本研究) | 水解 | R | 99.0 |
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
/
| 〈 |
|
〉 |