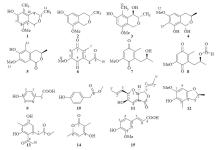
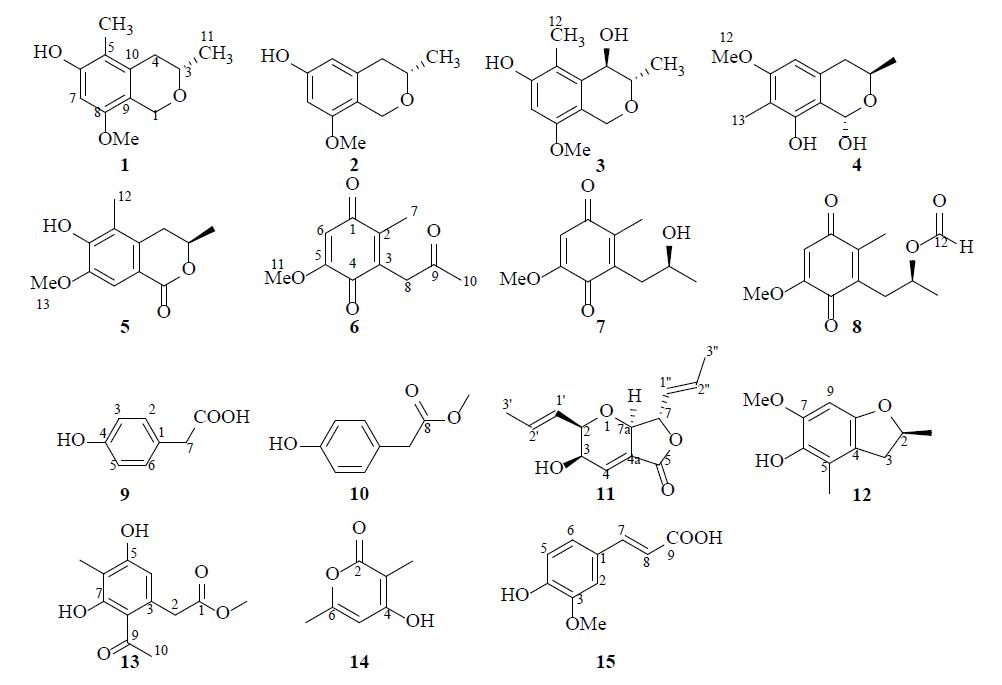
| [1] |
冯婷, 孙建, 王玉妃, 等, 2024. 北部湾海洋真菌Aspergillus fumigatus DL-p0m-g2的化学成分及药理活性研究[J]. 热带海洋学报, 43(1): 154-166.
|
|
FENG TING, SUN JIAN, WANG YUFEI, et al, 2024. Study on the chemical constituents and pharmacological activity of the marine-derived fungus Aspergillus fumigatus DL-p0m-g2 in the Beibu Gulf[J]. Journal of Tropical Oceanography, 43(1): 154-166 (in Chinese with English abstract).
|
| [2] |
何维彬, 高亮, 艾玉廷, 等, 2023. 羊栖菜化学成分及药理活性研究进展[J]. 中草药, 54(7): 2327-2337.
|
|
HE WEIBIN, GAO LIANG, AI YUTING, et al, 2023. Research progress on chemical constituents and pharmacological activities of Sargassum fusiforme[J]. Chinese Traditional and Herbal Drugs, 54(7): 2327-2337 (in Chinese with English abstract).
|
| [3] |
黄正, 霍会霞, 任易, 等, 2017. 大花益母草的化学成分研究[J]. 中草药, 48(9): 1724-1729.
|
|
HUANG ZHENG, HUO HUIXIA, REN YI, et al, 2017. Chemical constituents from aerial parts of Leonurus macranthus[J]. Chinese Traditional and Herbal Drugs, 48(9): 1724-1729 (in Chinese with English abstract).
|
| [4] |
刘媛媛, 闫芳, 章朦玥, 等, 2022. 荚果蕨植物内生真菌Penicillium sp. SYP-F-7610的抑菌活性次级代谢产物研究[J]. 中国抗生素杂志, 47(5): 501-506.
|
|
LIU YUANYUAN, YAN FANG, ZHANG MENGYUE, et al, 2022. Study on antibacterial secondary metabolites from the endophytic fungus Penicillium sp. SYP-F-7610 isolated from Matteuccia struthiopteris[J]. Chinese Journal of Antibiotics, 47(5): 501-506 (in Chinese with English abstract).
|
| [5] |
罗志宏, 钟雯雯, 刘昕明, 等, 2023. 隐板裸刺蛇尾共附生真菌的分离鉴定及其代谢产物的抑菌活性研究[J]. 广西科学, 30(3): 485-493.
|
|
LUO ZHIHONG, ZHONG WENWEN, LIU XINMING, et al, 2023. Isolation and identification of symbiotic or epiphytic fungi from Gymnolophus obscura and study on the antibacterial activity of their metabolites[J]. Guangxi Sciences, 30(3): 485-493 (in Chinese with English abstract).
|
| [6] |
王艳颖, 罗都强, 师宝忠, 等, 2011. 番石榴叶内生真菌Pestalotiopsis zonata次级代谢产物的研究[J]. 中成药, 33(5): 857-859 (in Chinese).
|
| [7] |
夏辰曦, 刘昕明, 彭亮, 等, 2023. 热浪岛珊瑚礁泥砂真菌多样性及真菌Cladosporium sp. GXIMD02067天然产物分离与鉴定[J]. 微生物学通报, 50(11): 4784-4795.
|
|
XIA CHENXI, LIU XINMING, PENG LIANG, et al, 2023. Fungal diversity in coral reef sand of Redang Island and separation and identification of the natural products from Cladosporium sp. GXIMD02067[J]. Microbiology China, 50(11): 4784-4795 (in Chinese with English abstract).
|
| [8] |
邢楠楠, 任润馨, 唐振洲, 等, 2023. 涠洲岛海洋沉积物来源真菌Aspergillus sp. GXIMD02003的代谢产物研究[J]. 热带海洋学报, 42(5): 154-160.
|
|
XING NANNAN, REN RUNXIN, TANG ZHENZHOU, et al, 2023. Study on the secondary metabolites of fungus Aspergillus sp. GXIMD02003 derived from marine sediment in the Weizhou island[J]. Journal of Tropical Oceanography, 42(5): 154-160 (in Chinese with English abstract).
|
| [9] |
叶禹秀, 罗小卫, 杨斌, 等, 2022. 南海礁栖海藻共附生真菌Pestalotiopsis neglecta SCSIO41403次级代谢产物研究[J]. 热带海洋学报, 41(3): 186-190.
|
|
YE YUXIU, LUO XIAOWEI, YANG BIN, et al, 2022. Study on the secondary metabolites of reef habitat algae-derived fungus Pestalotiopsis neglecta SCSIO41403 from the South China Sea[J]. Journal of Tropical Oceanography, 41(3): 186-190 (in Chinese with English abstract).
|
| [10] |
DRAMAE A, INTARAUDOM C, BUNBAMRUNG N, et al, 2022. Antimicrobial tanzawaic acid derivatives from the endophytic Penicillium citrinum BCC71086[J]. Tetrahedron, 106-107: 132645.
|
| [11] |
EL-ELIMAT T, FIGUEROA M, RAJA H A, et al, 2013. Waol A, trans-dihydrowaol A, and cis-dihydrowaol A: polyketide-derived γ-lactones from a Volutella species[J]. Tetrahedron Letters, 54(32): 4300-4302.
|
| [12] |
GAUTSCHI J T, AMAGATA T, AMAGATA A, et al, 2004. Expanding the strategies in natural product studies of marine-derived fungi: A chemical investigation of Penicillium obtained from deep water sediment[J]. Journal of Natural Products, 67(9): 362-367.
|
| [13] |
HAMMERSCHMIDT L, DEBBAB A, NGOC T D, et al, 2014. Polyketides from the mangrove-derived endophytic fungus Acremonium strictum[J]. Tetrahedron Letters, 55(24): 3463-3468.
|
| [14] |
LI KE, CHUNG-DAVIDSON Y W, BUSSY U, et al, 2015. Recent advances and applications of experimental technologies in marine natural product research[J]. Marine Drugs, 13(5): 2694-2713.
|
| [15] |
MIAO FENGPING, LIANG XIAORUI, LIU XIANGHONG, et al, 2014. Aspewentins A-C, norditerpenes from a cryptic pathway in an algicolous strain of Aspergillus wentii[J]. Journal of Natural Products, 77(2): 429-432.
|
| [16] |
NICOLETTI R, TRINCONE A, 2016. Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin[J]. Marine Drugs, 14(2): 37.
|
| [17] |
NOZAWA O, OKAZAKI T, MORIMOTO S, et al, 2000. Waol B, a new trihydrofuran derivative with cytocidal activity, isolated from Myceliophthora lutea[J]. The Journal of Antibiotics, 53(11): 1296-1300.
|
| [18] |
OGAWA A, MURAKAMI C, KAMISUKI S, et al, 2004. Pseudodeflectusin, a novel isochroman derivative from Aspergillus pseudodeflectus a parasite of the sea weed, Sargassum fusiform, as a selective human cancer cytotoxin[J]. Bioorganic & Medicinal Chemistry Letters, 14(13): 3539-3543.
|
| [19] |
ORFALI R S, ALY A H, EBRAHIM W, et al, 2015. Isochroman and isocoumarin derivatives from hypersaline lake sediment-derived fungus Penicillium sp.[J]. Phytochemistry Letters, 13: 234-238.
|
| [20] |
QUANG T H, NHIEM N X, TAI B H, et al, 2018. Secondary metabolites from the marine-derived fungus Paraconiothyrium sp. VK-13[J]. Vietnam Journal of Chemistry, 56(4): 434-439.
|
| [21] |
SMETANINA O F, YURCHENKO A N, IVANETS E V, et al, 2016. Metabolites of the marine fungus Penicillium citrinum associated with a brown alga Padina sp.[J]. Chemistry of Natural Compounds, 52(1): 111-112.
|
| [22] |
SMETANINA O F, YURCHENKO A N, IVANETS E V, et al, 2017. Aromatic metabolites of marine fungus Penicillium sp. KMM 4672 associated with a brown alga Padina sp.[J]. Chemistry of Natural Compounds, 53(3): 600-602.
|
| [23] |
WANG HUIJUAN, GLOER K B, GLOER J B, et al, 1997. Anserinones A and B: new antifungal and antibacterial benzoquinones from the coprophilous fungus Podospora anserina[J]. Journal of Natural Products, 60(6): 629-631.
|
| [24] |
XU JIANLIN, LIU HONGXIN, CHEN YUCHAN, et al, 2018. Highly substituted benzophenone aldehydes and eremophilane derivatives from the deep-sea derived fungus Phomopsis lithocarpus FS508[J]. Marine Drugs, 16(9): 329.
|
| [25] |
ZHANG LIJUAN, YANG MINGFEI, MA JIAN, et al, 2023. Neogrisphenol A, a potential ovarian cancer inhibitor from a new record fungus Neohelicosporium griseum[J]. Metabolites, 13(3): 435.
|
 ), JIN Xin, LIU Yonghong, GAO Chenghai, CHEN Xianqiang(
), JIN Xin, LIU Yonghong, GAO Chenghai, CHEN Xianqiang( )
)