Journal of Tropical Oceanography ›› 2024, Vol. 43 ›› Issue (4): 20-32.doi: 10.11978/2023117CSTR: 32234.14.2023117
• Review • Previous Articles Next Articles
Geochemistry of black carbon in marine extreme environments and its environmental implications*
LI Dai( ), WANG Xudong(
), WANG Xudong( ), JIA Zice, FENG Dong
), JIA Zice, FENG Dong
- College of Oceanography and Ecological Science, Shanghai Ocean University, Shanghai 201306, China
-
Received:2023-08-12Revised:2023-09-08Online:2024-07-10Published:2024-07-22 -
Supported by:National Natural Science Foundation of China(42106059); Shanghai Sailing Program(21YF1416800); Shanghai Chenguang Program(22CGA58)
Cite this article
LI Dai, WANG Xudong, JIA Zice, FENG Dong. Geochemistry of black carbon in marine extreme environments and its environmental implications*[J].Journal of Tropical Oceanography, 2024, 43(4): 20-32.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
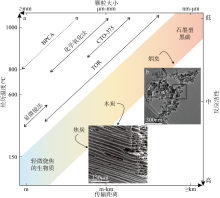
Fig. 1
Physicochemical properties and extraction methods of black carbon. (a) The solid line shows the range of applicability of the extraction method and the dashed line represents the range of applicability that is not clear yet. TOR is thermal-optical reflectance. CTO-375 is thermal oxidation at 375℃ for 24h. BPCA is benzene polycarboxylic acid molecular marker method; (b) TEM image of soot; (c) SEM image of char (modified from Masiello, 2004; Hammes et al, 2007; Buseck et al, 2012; Bird et al, 2015)"
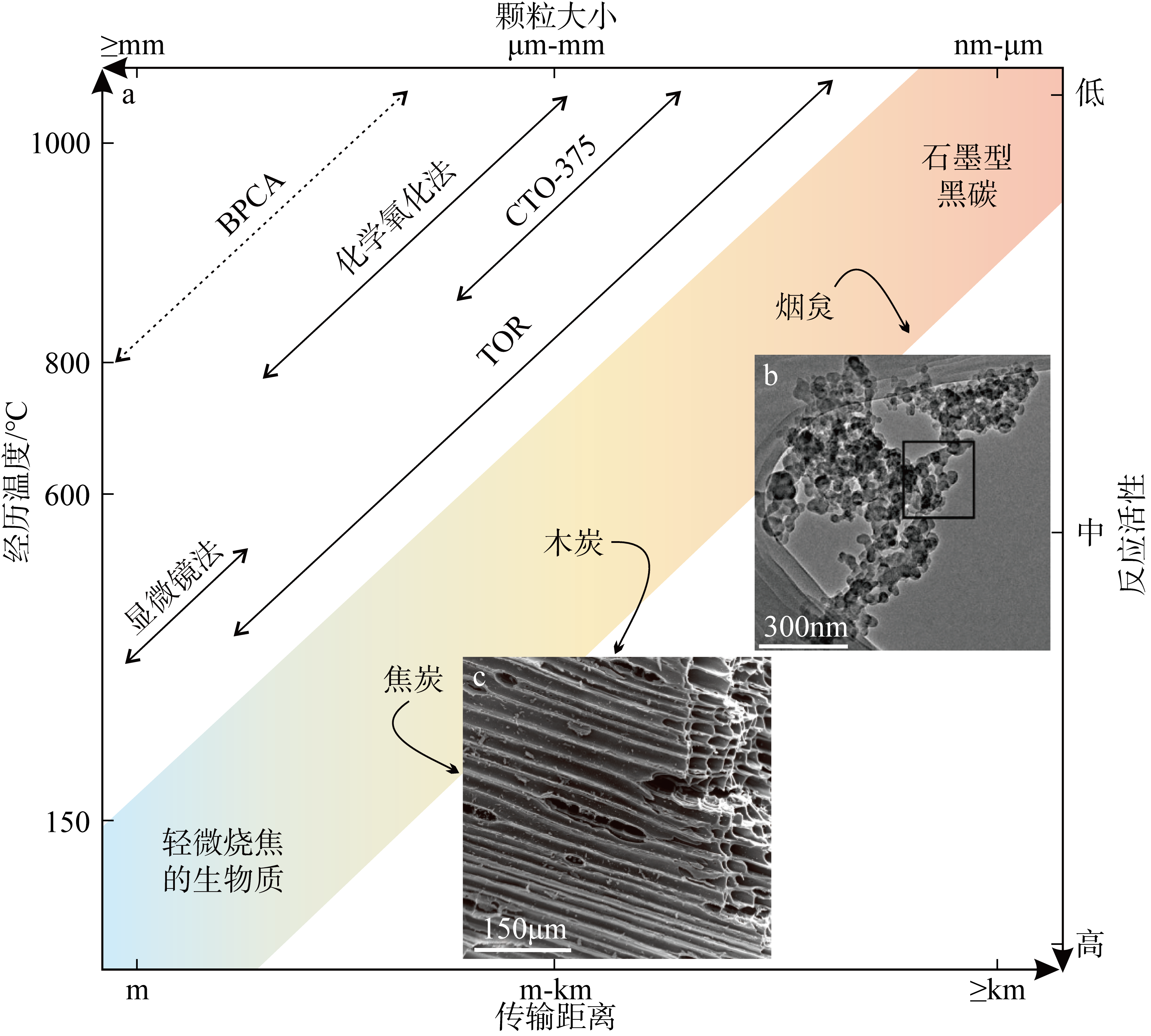
Fig. 2
Global compilation of marine sediment black carbon content (data from Smith et al, 1973; Griffin et al, 1975; Lim et al, 1996; Gustafsson et al, 1998; Kang et al, 2009; Lohmann et al, 2009; Salvadó et al, 2017; Yang et al, 2018; Ren et al, 2019, 2022; Wu et al, 2019; Dan et al, 2022; Wulandari et al, 2023)"

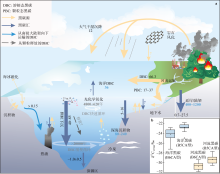
Fig. 3
Marine black carbon cycle. (a) The black value represents the annual flux of black carbon (Tg·yr−1), the blue value represents the total storage of black carbon (Pg); (b) it is the δ13C values representing different types of black carbon extracted by the BPCA method; the oxidation of DBC by concentrated nitric acid under high-temperature and high-pressure conditions results in the formation of benzene polyacid (BPCA, B3CA-B6CA) compounds, which contain 3~6 carboxyl groups; the monomer concentration ratio of benzene polyacid is influenced by the structure and source of DBC; therefore, the stable carbon isotope (δ13C) values of specific DBCs, such as benzene pentapolyacid (B5CA) and benzene hexapolyacid (B6CA), can be utilized to track the origin and formation process of BC; furthermore, the B6CA:B5CA ratio is a widely employed index for assessing the aromaticity of DBC; a higher value of this ratio indicates a greater degree of aromaticity in DBC (modified from Fang, 2018; Wagner et al, 2019)"

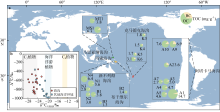
Fig. 4
Geochemistry of black carbon in trench sediments. (a) The area of the pie chart indicates the TOC content of each site, green in the pie chart represents the organic carbon content except black carbon, yellow represents the black carbon content, the ‘letter+number’ next to the pie chart represents the station number, and the ‘number’ next to the pie chart represents the TOC content of the station in mg·g−1; (b) cross-plot of δ13C and Δ14C for the black carbon (BC) in trench sediments and other marine sediments (modified from Zhang et al, 2022)"

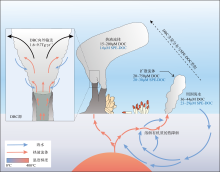
Fig. 5
Schematic diagram of dissolved organic carbon changes in the hydrothermal systems. SPE-DOC (solid phase extraction-DOC) represents recalcitrant dissolved organic carbon extracted by solid phase extraction, and about 94% of SPE-DOC is removed at hydrothermal vents compared to surrounding seawater (modified from Hawkes et al, 2015; Niggemann et al, 2016; Dick, 2019)"

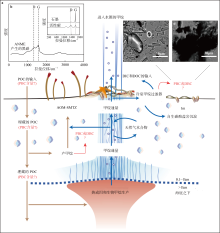
Fig. 6
(a) Carbon cycle of cold seeps, sulfate-methane transition zone (SMTZ); (b) Raman spectroscopic characterization of the black carbon in methane anaerobic oxidizing archaea (ANME), activated carbon, and graphite. (c) and (d) the black spherical material is the black carbon produced by ANME (modified from Boetius et al, 2013; Allen et al, 2021)"
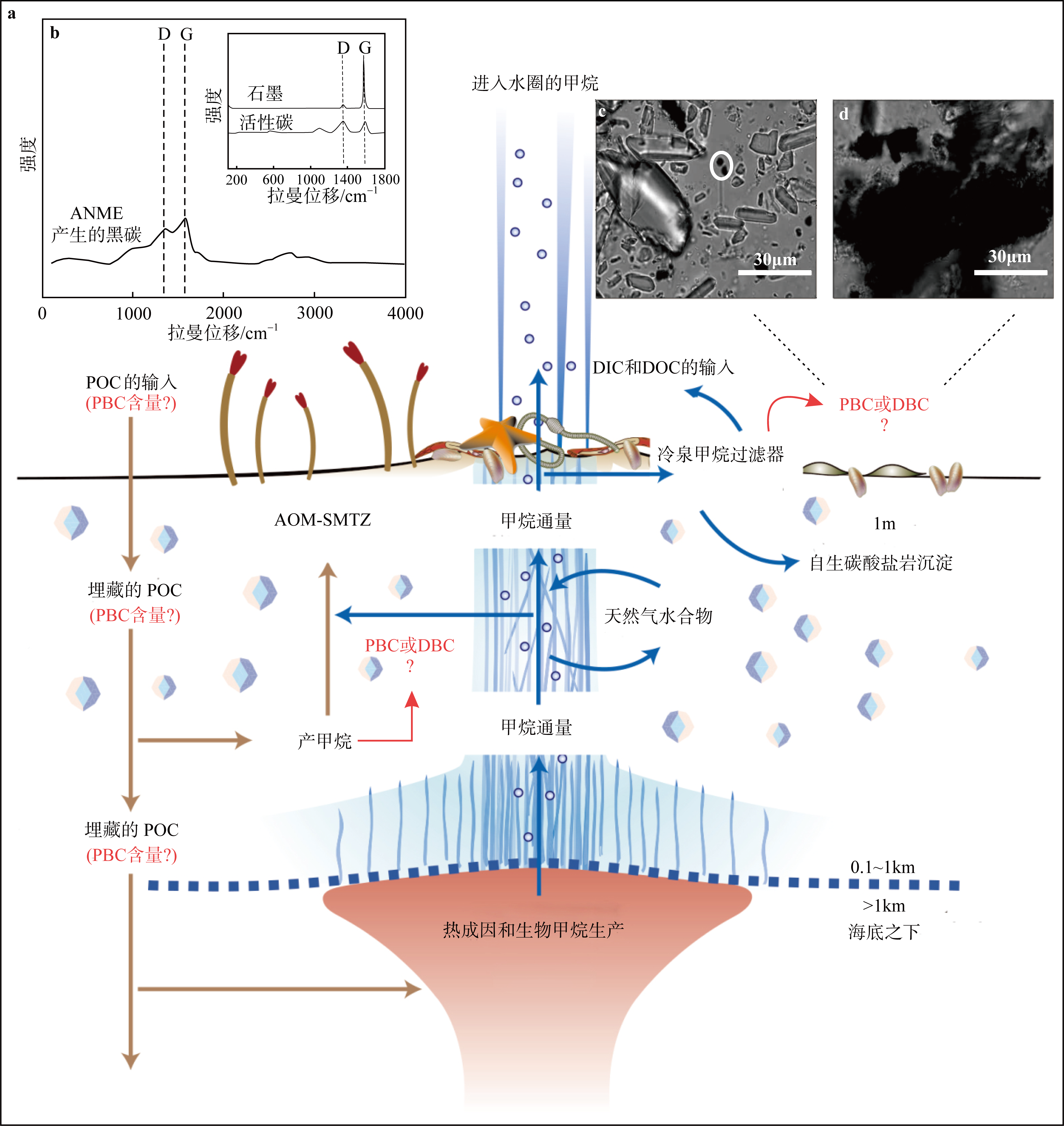

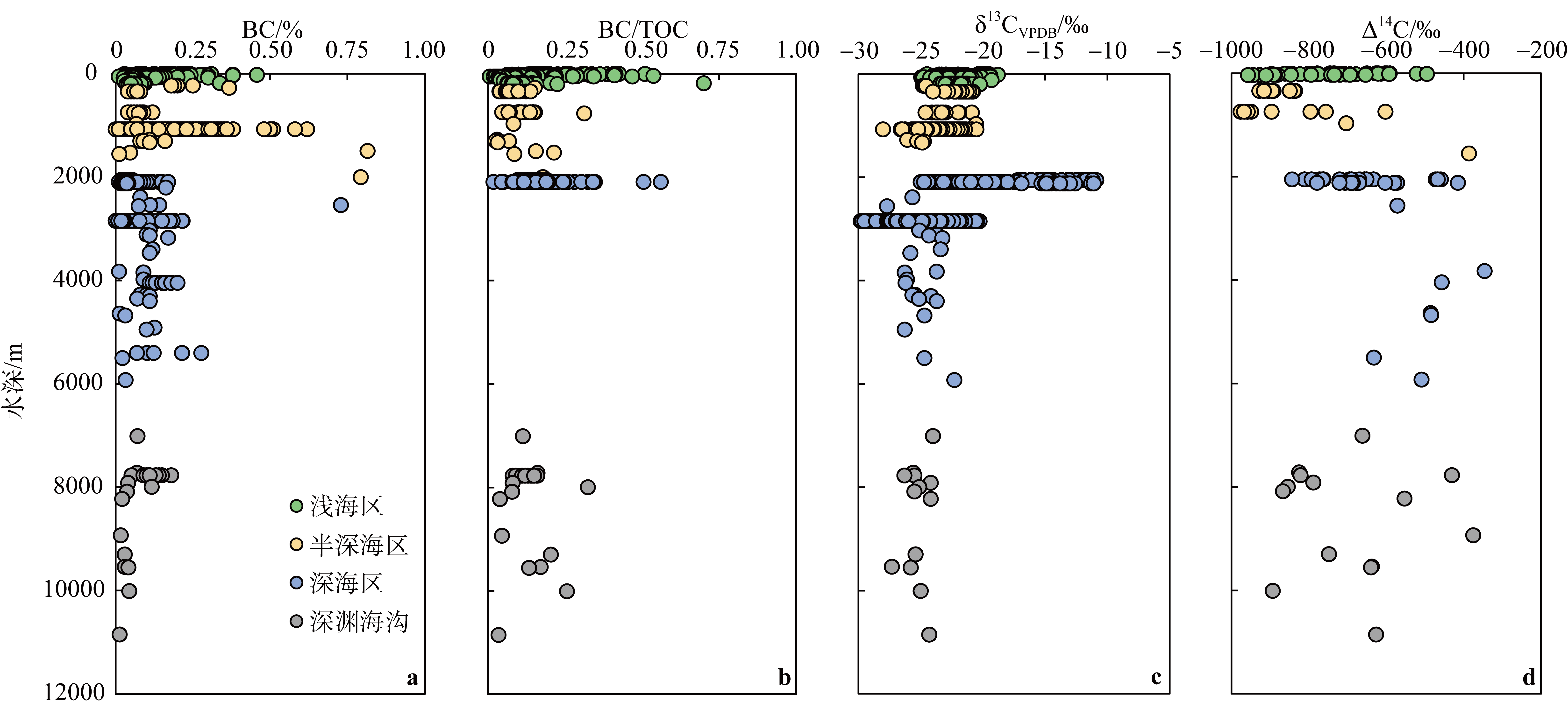
Fig. 7
Geochemical characteristics of BC in different depth profiles of marine sediments. According to the depth of seawater: shallow sea (0~200m), semi-deep sea (200~2000m), deep sea (2000~6000m) and hadal zone (> 6000m). (a) The content of BC in the sediment; (b) the BC/TOC value in the sediment; (c) the δ13C value of BC in the sediment; (d) the Δ14C value of BC in the sediment (the data in the figure are cited from Middelburg et al, 1999; Kang et al, 2009; Lohmann et al, 2009; Li et al, 2012; Salvadó et al, 2017; Zhou et al, 2017; Shen et al, 2018; Yang et al, 2018; Ren et al, 2019, 2022; Wu et al, 2019; Pei et al, 2020; Dan et al, 2022; Liu et al, 2022; Zhang et al, 2022; Fu et al, 2023; Li et al, 2023a; Wulandari et al, 2023)"
| [1] |
曹军骥, 占长林, 2011. 黑碳在全球气候和环境系统中的作用及其在相关研究中的意义[J]. 地球科学与环境学报, 33(2): 177-184.
|
|
|
|
| [2] |
方仔铭, 2018. 南海及极地海域黑碳的源、汇及其对海洋碳循环的影响[D]. 厦门: 厦门大学: 1-109.
|
|
|
|
| [3] |
夏翠梅, 王楠, 刘景昱, 等, 2023. “碳中和”目标下海洋黑碳的源汇过程及其意义[J/OL]. 地质科技通报. https://doi.org/10.19509/j.cnki.dzkq.tb20220455.
|
|
|
|
| [4] |
徐翠玲, 孙治雷, 吴能友, 等, 2020. 海底泥火山的甲烷迁移与转化及其对海洋碳输入的影响[J]. 海洋地质与第四纪地质, 40(6): 1-13.
|
|
|
|
| [5] |
赵维殳, 肖湘, 2023. 深海热液区微生物群落与环境适应性机理[J]. 中国科学: 生命科学, 53(5): 660-671.
|
|
|
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
doi: 10.1038/s41561-018-0159-8 |
| [15] |
|
| [16] |
|
| [17] |
doi: 10.1038/s41579-019-0160-2 pmid: 30867583 |
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
doi: 10.1038/s41467-019-13216-z pmid: 31729377 |
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
doi: 10.1038/NGEO2543 |
| [32] |
|
| [33] |
|
| [34] |
doi: 10.1038/s41467-020-16576-z pmid: 32494057 |
| [35] |
|
| [36] |
|
| [37] |
doi: 10.1146/annurev.micro.61.080706.093130 pmid: 19575572 |
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
doi: 10.1038/s41467-020-18808-8 pmid: 33028806 |
| [63] |
doi: 10.1016/j.scitotenv.2019.06.437 |
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
doi: 10.1038/s41467-019-13111-7 pmid: 31699996 |
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
doi: 10.1038/s41467-022-27954-0 pmid: 35027558 |
| [79] |
|
| [80] |
doi: 10.1038/s41467-020-17860-8 pmid: 32770005 |
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| No related articles found! |
|
||

