Journal of Tropical Oceanography ›› 2023, Vol. 42 ›› Issue (5): 45-55.doi: 10.11978/2022236CSTR: 32234.14.2022236
• Marine Chemistry • Previous Articles Next Articles
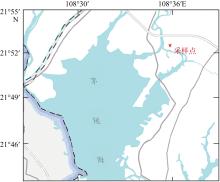
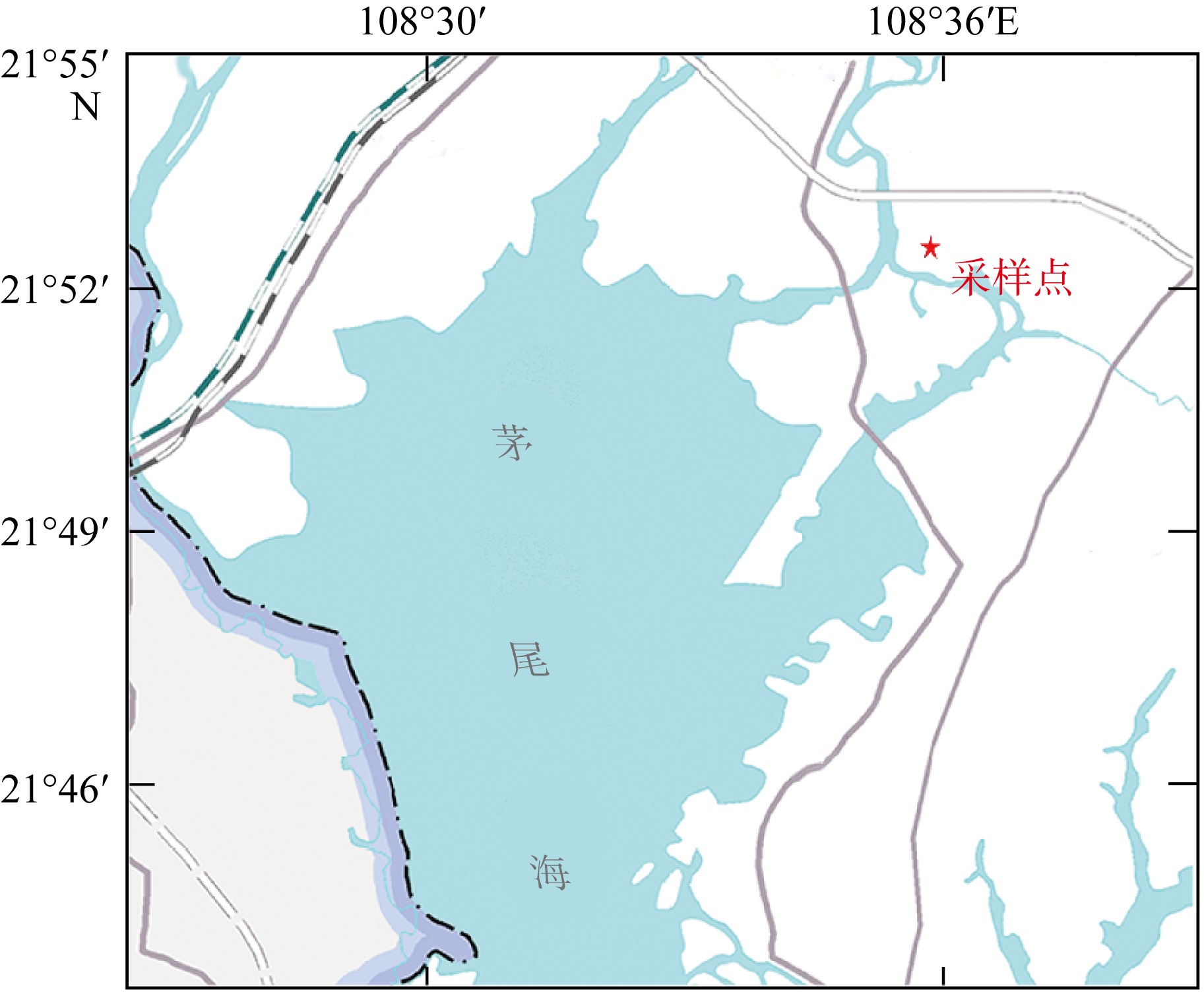
Coupling characteristics of reduced inorganic sulfur and reactive iron in coastal acidic sulfate soil wetland and its environmental significance
CHEN Bo1,2( ), QIN Zidong1, WANG Feng1,2, CAI Pingxiong1,2, ZHANG Shengyin3
), QIN Zidong1, WANG Feng1,2, CAI Pingxiong1,2, ZHANG Shengyin3
- 1. College of Petroleum and Chemical Engineering, Beibu Gulf University, Qinzhou 535000, China
2. Guangxi Key Laboratory of Green Chemical Materials and Safety Technology, Qinzhou 535000, China
3. Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou 730000, China
-
Received:2022-11-07Revised:2023-01-17Online:2023-09-10Published:2023-02-22 -
Supported by:Guangxi Natural Science Foundation(2020GXNSFBA297128); Special Talent Project of Guangxi Science and Technology Base(Guike AD20238041); Cultivation Project Jointly funded by Guangxi Natural Science Foundation(2019GXNSFAA245016)
Cite this article
CHEN Bo, QIN Zidong, WANG Feng, CAI Pingxiong, ZHANG Shengyin. Coupling characteristics of reduced inorganic sulfur and reactive iron in coastal acidic sulfate soil wetland and its environmental significance[J].Journal of Tropical Oceanography, 2023, 42(5): 45-55.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
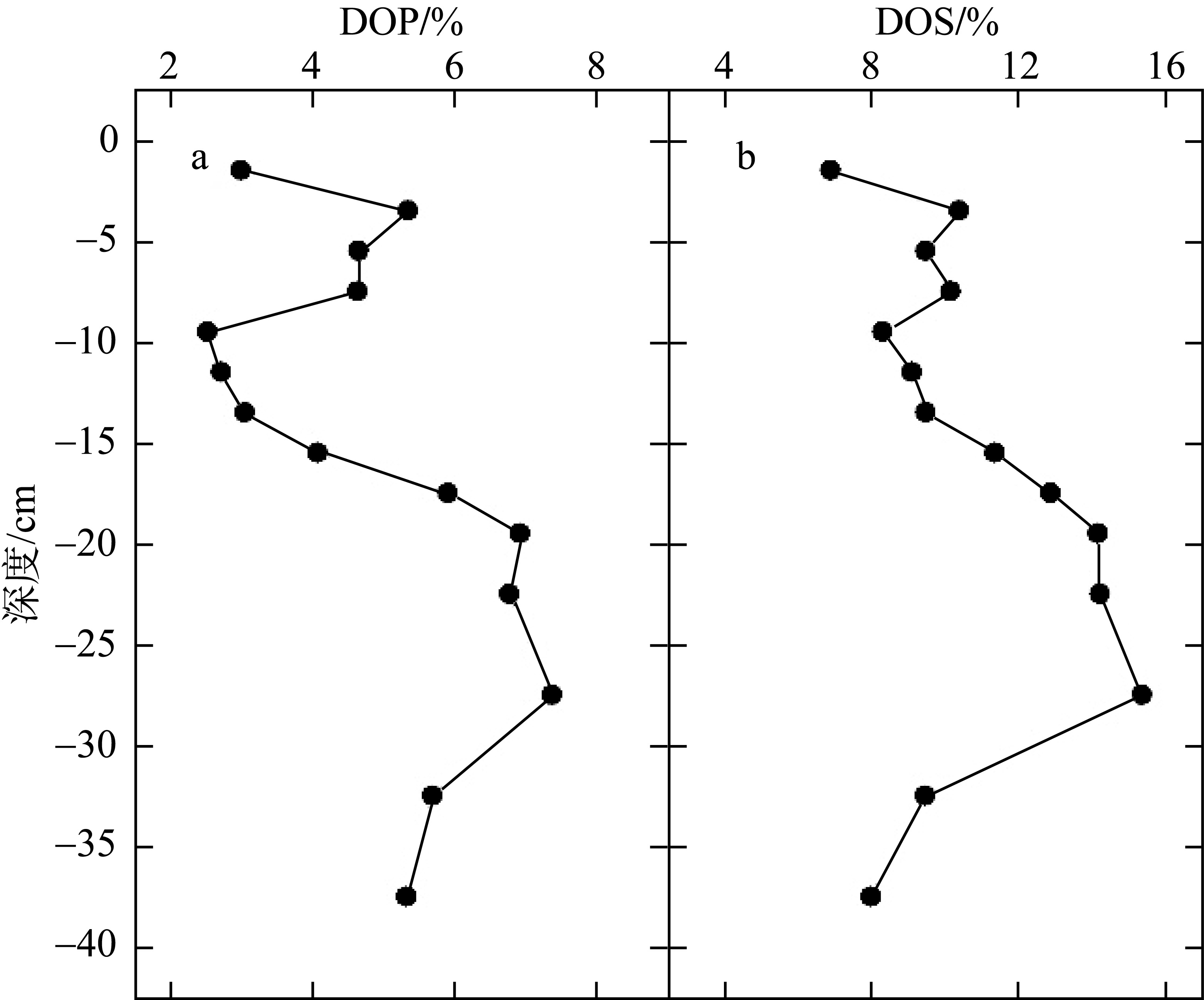
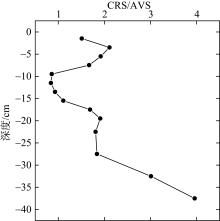
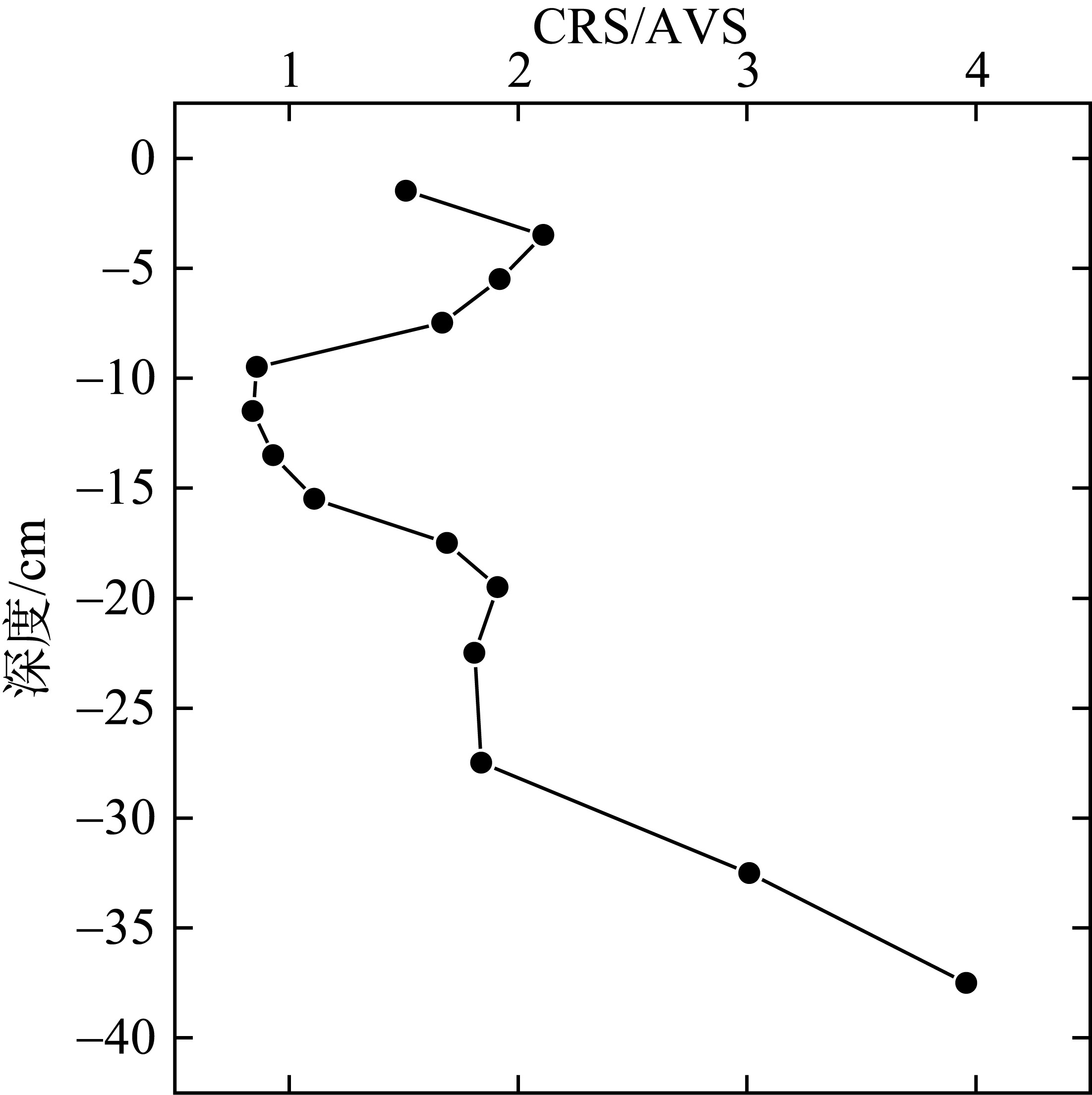
Tab. 1
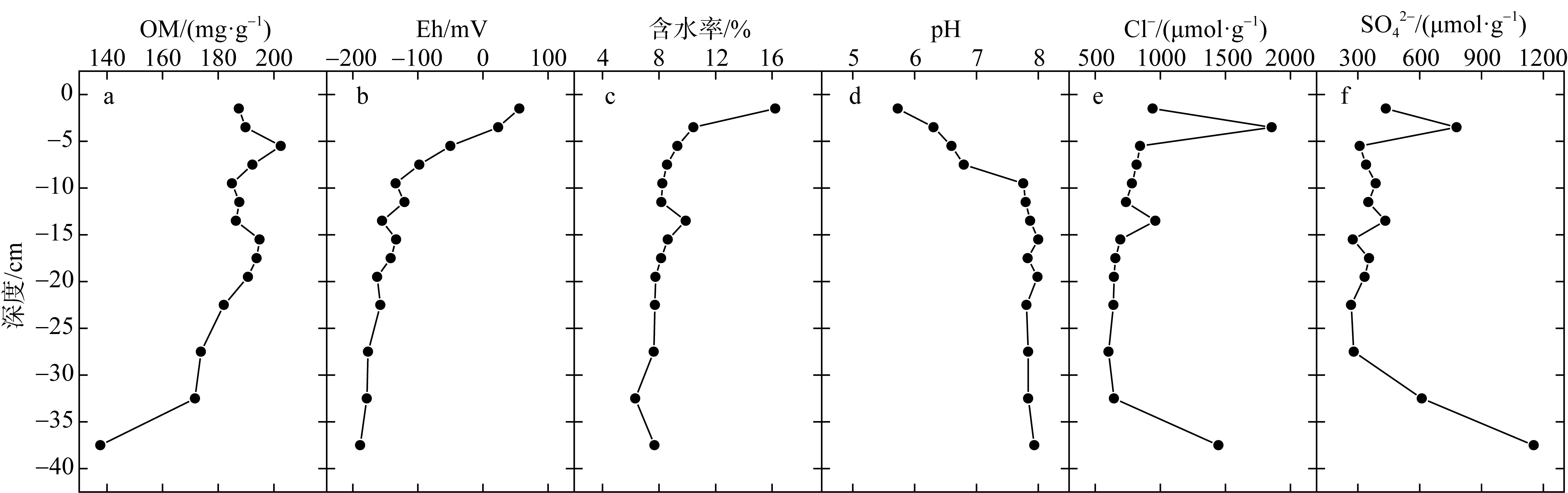
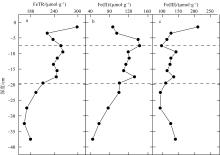
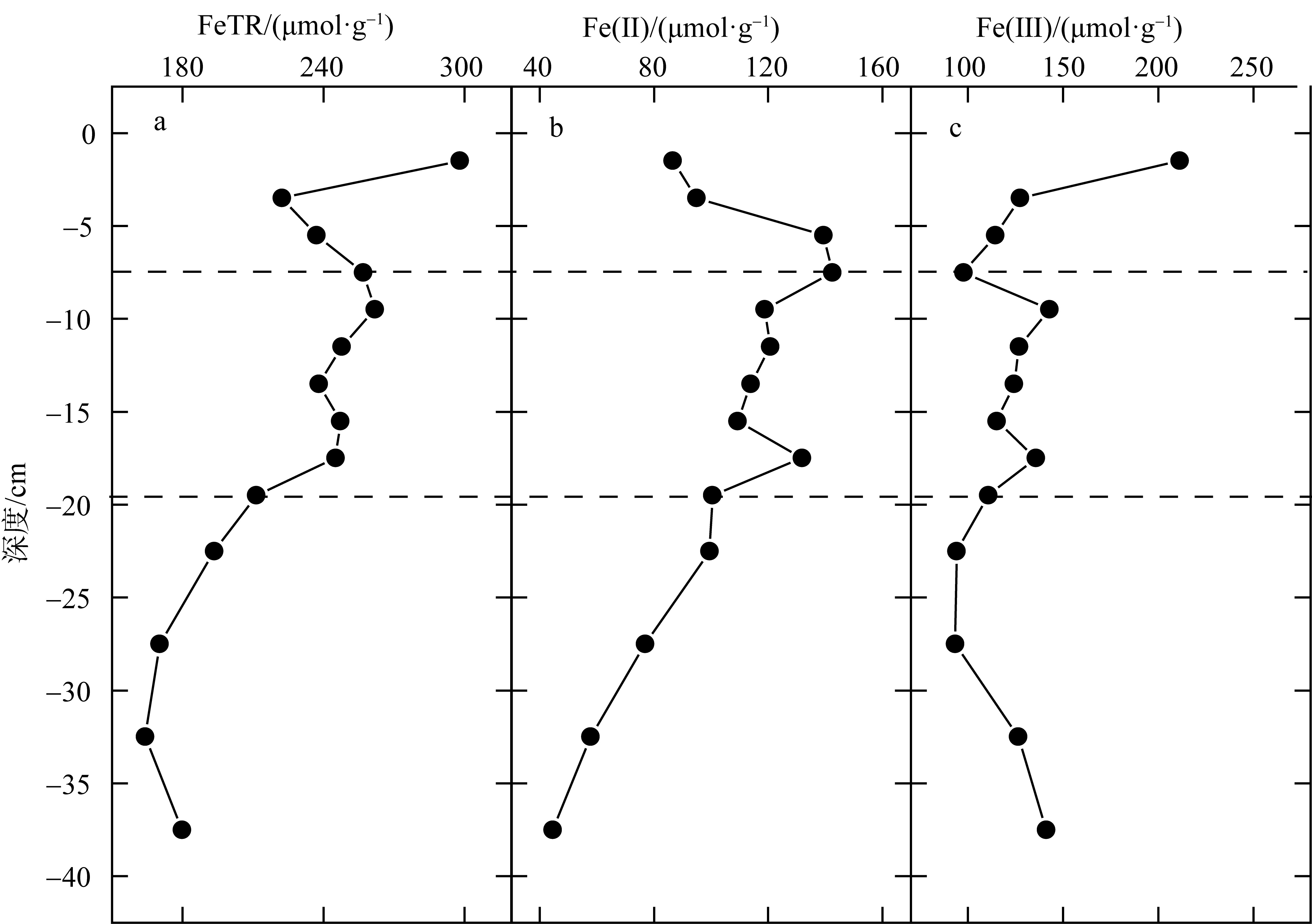
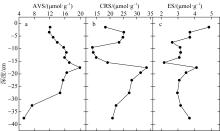
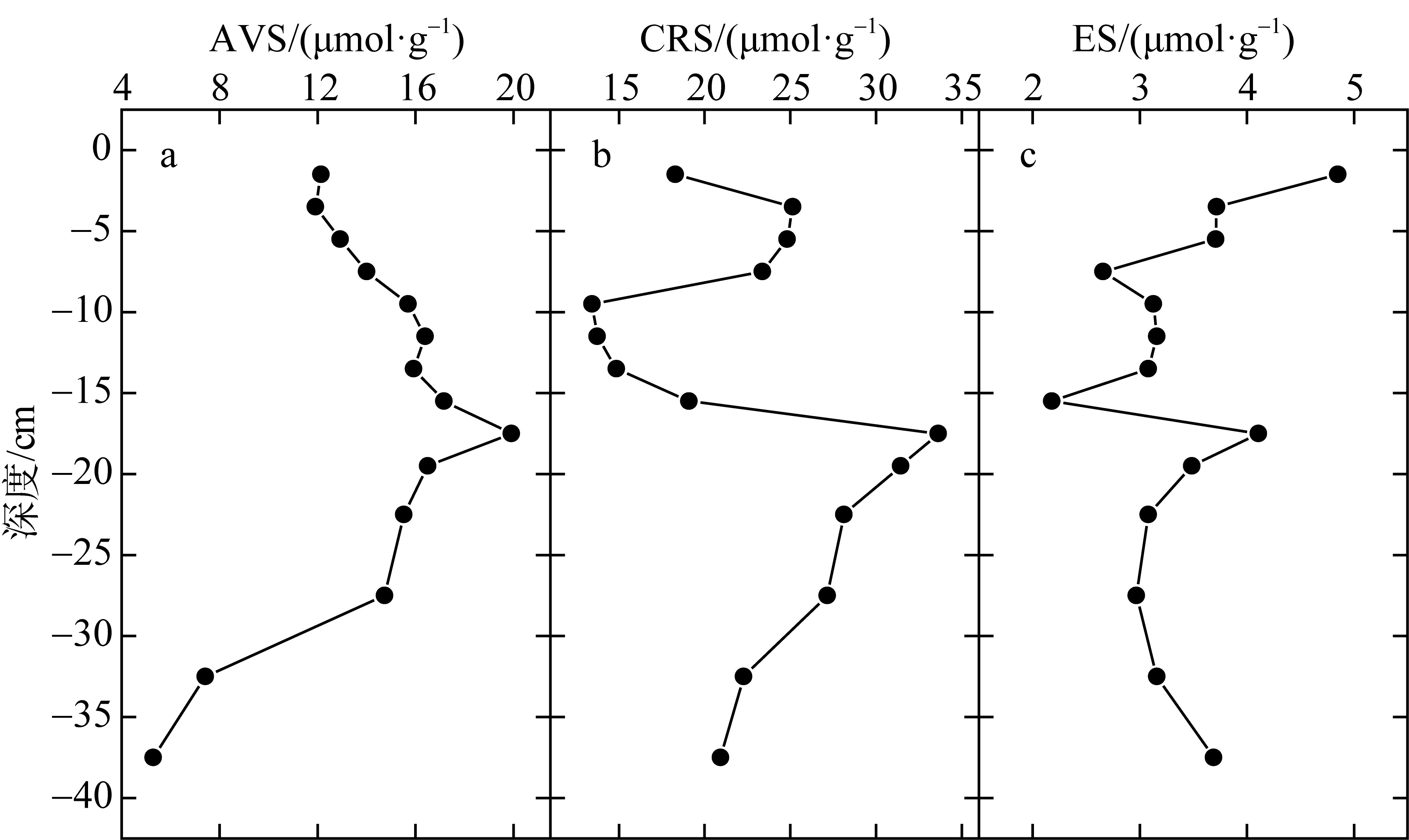
Correlation analysis of active iron and environmental factors and RIS in the sediment"
| FeTR | Fe(Ⅱ) | Fe(Ⅲ) | OM | Eh | AVS | CRS | ES | RIS | pH | 含水率 | Cl- | SO42- | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FeTR | 1 | ||||||||||||
| Fe(Ⅱ) | 0.643* | 1 | |||||||||||
| Fe(Ⅲ) | 0.545* | -0.215 | 1 | ||||||||||
| OM | 0.600* | 0.832** | -0.097 | 1 | |||||||||
| Eh | 0.630* | 0.227 | 0.562* | 0.442 | 1 | ||||||||
| AVS | 0.444 | 0.730** | -0.233 | 0.716** | -0.05 | 1 | |||||||
| CRS | -0.383 | 0.01 | -0.372 | 0.111 | -0.116 | 0.168 | 1 | ||||||
| ES | 0.271 | -0.153 | 0.742** | -0.05 | 0.556* | -0.199 | 0.197 | 1 | |||||
| RIS | 0.044 | 0.463 | -0.328 | 0.587* | -0.006 | 0.684** | 0.801** | 0.093 | 1 | ||||
| pH | -0.521 | -0.172 | -0.499 | -0.332 | -0.948** | 0.213 | 0.046 | -0.563* | 0.039 | 1 | |||
| 含水率 | 0.690** | 0.069 | 0.771** | 0.26 | 0.841** | -0.013 | -0.255 | 0.629* | -0.118 | -0.798** | 1 | ||
| Cl- | 0.012 | -0.276 | 0.260 | -0.299 | 0.468 | -0.498 | -0.128 | 0.31 | -0.438 | -0.44 | 0.298 | 1 | |
| SO42- | -0.345 | -0.664** | 0.294 | -0.766** | 0.009 | -0.797** | -0.118 | 0.288 | -0.621* | -0.059 | -0.015 | 0.775** | 1 |
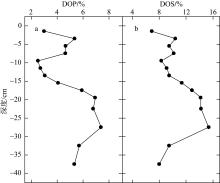
Tab. 2
Comparison of CRS/AVS, DOP and DOS between the study area and other study areas at home and abroad"
| 地点 | CRS/AVS | DOP/% | DOS/% | 数据来源 |
|---|---|---|---|---|
| 钦江河口 | 0.93~3.00 | 2.5~7.4 | 6.1~15.4 | 本研究 |
| 澳大利亚东海岸乔治湖 | — | 0.6~77.8 | 5.0~79.3 | Schoepfer等( |
| 烟台夹河口 | 0.99~11.4 | 1~24 | 3~25 | 姜明等( |
| 黄海胶州湾 | 0.28~5.39 | 10~36 | 20~103 | Zhu等( |
| 墨西哥湾 | 1.25~10.38 | 56~95 | 63~266 | Morse等( |
| 黑海 | — | 80~95 | 40~80 | Yücel等(2010) |
| [1] |
曹爱丽, 周桂平, 胡姝, 等, 2010. 崇明东滩湿地沉积物中还原无机硫的形态特征[J]. 复旦学报(自然科学版), 49(5): 612-617.
|
|
|
|
| [2] |
丰卫华, 王志富, 张荣保, 等, 2016. 宁德海域表层沉积物氧化还原环境特征及其影响因素[J]. 海洋环境科学, 35(6): 882-887.
|
|
|
|
| [3] |
国家环境保护总局, 2002. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社: 368-370.
|
|
北京:中国环境科学出版社: 368-370. THE STATE ENVIRONMENTAL PROTECTION ADMINISTRATION, 2002. Water and wastewater monitoring and analysis method[M]. Fourth Edition. Beijing: China Environmental Science Press: 368-370 (in Chinese with English abstract).
|
|
| [4] |
黄巧义, 唐拴虎, 卢瑛, 等, 2014. 酸性硫酸盐土的形成、特性及其生态环境效应[J]. 植物营养与肥料学报, 20(6): 1534-1544.
|
|
|
|
| [5] |
黄旭, 唐拴虎, 杨少海, 等, 2015. 酸性硫酸盐土壤改良对不同品种水稻生育性状的影响[J]. 中国土壤与肥料, (6): 48-56.
|
|
|
|
| [6] |
姜明, 赵国强, 李兆冉, 等, 2018. 烟台夹河口外柱状沉积物还原性无机硫、活性铁的变化特征及其相互关系[J]. 海洋科学, 42(8): 90-97.
|
|
|
|
| [7] |
吕仁燕, 朱茂旭, 李铁, 等, 2011. 东海陆架泥质沉积物中固相Fe形态及其对有机质、Fe、S成岩路径的制约意义[J]. 地球化学, 40(4): 363-371.
|
|
|
|
| [8] |
钱宝, 刘凌, 肖潇, 2011. 土壤有机质测定方法对比分析[J]. 河海大学学报(自然科学版), 39(1): 34-38.
|
|
|
|
| [9] |
施玉珍, 张际标, 李雪英, 等, 2012. 湛江湾沉积物中酸可挥发性硫和重金属含量分布及重金属生态风险评价[J]. 应用海洋学学报, 31(4): 466-471.
|
|
|
|
| [10] |
孙启耀, 2016. 河口沉积物硫的地球化学特征及其与铁和磷的耦合机制初步研究[D]. 烟台: 中国科学院烟台海岸带研究所.
|
|
|
|
| [11] |
唐罗忠, 生原喜久雄, 户田浩人, 等, 2005. 湿地林土壤的Fe2+, Eh及pH值的变化[J]. 生态学报, (1): 103-107.
|
|
|
|
| [12] |
尹洪斌, 范成新, 丁士明, 等, 2008. 太湖梅梁湾与五里湖沉积物活性硫和重金属分布特征及相关性研究[J]. 环境科学, 29(7): 1791-1796.
|
|
|
|
| [13] |
于雯泉, 钟少军, 蒲晓强, 等, 2009. 胶州湾李村河河口区沉积物有机碳、酸可挥发硫化物及重金属元素的环境响应[J]. 古地理学报, 11(3): 338-347.
|
|
|
|
| [14] |
张伯虎, 陈沈良, 刘焱雄, 等, 2011. 广西钦州湾海域表层沉积物分异特征与规律[J]. 热带海洋学报, 30(4): 66-70.
|
|
doi: 10.11978/j.issn.1009-5470.2011.04.066 |
|
| [15] |
章家恩, 1999. 酸性硫酸盐土的酸害暴发机制及其环境影响[J]. 热带地理, 19(2): 137-141.
|
|
|
|
| [16] |
张璐, 2014. 胶州湾沉积物中硫酸盐还原和铁异化还原的影响因素研究[D]. 青岛: 中国海洋大学.
|
|
|
|
| [17] |
doi: 10.1007/s11356-016-6189-0 |
| [18] |
doi: 10.1016/S0009-2541(99)00198-9 |
| [19] |
doi: S0045-6535(14)00978-3 pmid: 25189685 |
| [20] |
pmid: 11695115 |
| [21] |
doi: 10.1016/j.geoderma.2009.04.015 |
| [22] |
doi: 10.1016/j.geoderma.2017.07.006 |
| [23] |
doi: 10.1016/j.chemgeo.2014.09.009 |
| [24] |
doi: 10.1016/0016-7037(95)00163-T |
| [25] |
doi: 10.1038/ismej.2015.50 |
| [26] |
doi: 10.1016/j.chemgeo.2016.02.013 |
| [27] |
doi: 10.1016/j.geoderma.2016.09.030 |
| [28] |
doi: S0045-6535(18)30113-9 pmid: 29407844 |
| [29] |
doi: 10.1016/0016-7037(85)90261-3 |
| [30] |
doi: 10.1016/j.gca.2013.08.013 |
| [31] |
doi: 10.1016/j.gca.2012.11.025 |
| [32] |
doi: 10.1007/s11430-015-5182-7 |
| [33] |
doi: 10.1016/j.gca.2020.06.003 |
| [34] |
doi: 10.1016/j.envint.2009.07.002 |
| [35] |
pmid: 15262162 |
| [36] |
doi: 10.1038/nrmicro3347 pmid: 25329406 |
| [37] |
doi: 10.1007/s10498-007-9012-1 |
| [38] |
doi: 10.1007/s10661-013-3108-4 pmid: 23355026 |
| [39] |
|
| [40] |
doi: 10.1016/j.marchem.2005.08.004 |
| [41] |
|
| [42] |
doi: 10.1039/c0em00589d pmid: 21267498 |
| [43] |
doi: 10.1016/j.apgeochem.2004.04.004 |
| [44] |
doi: 10.1016/j.quaint.2016.06.003 |
| [45] |
doi: 10.1016/j.chemgeo.2009.10.010 |
| [46] |
doi: 10.1016/j.marenvres.2012.06.010 |
| No related articles found! |
|
||