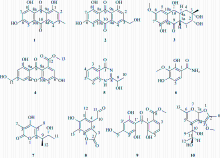
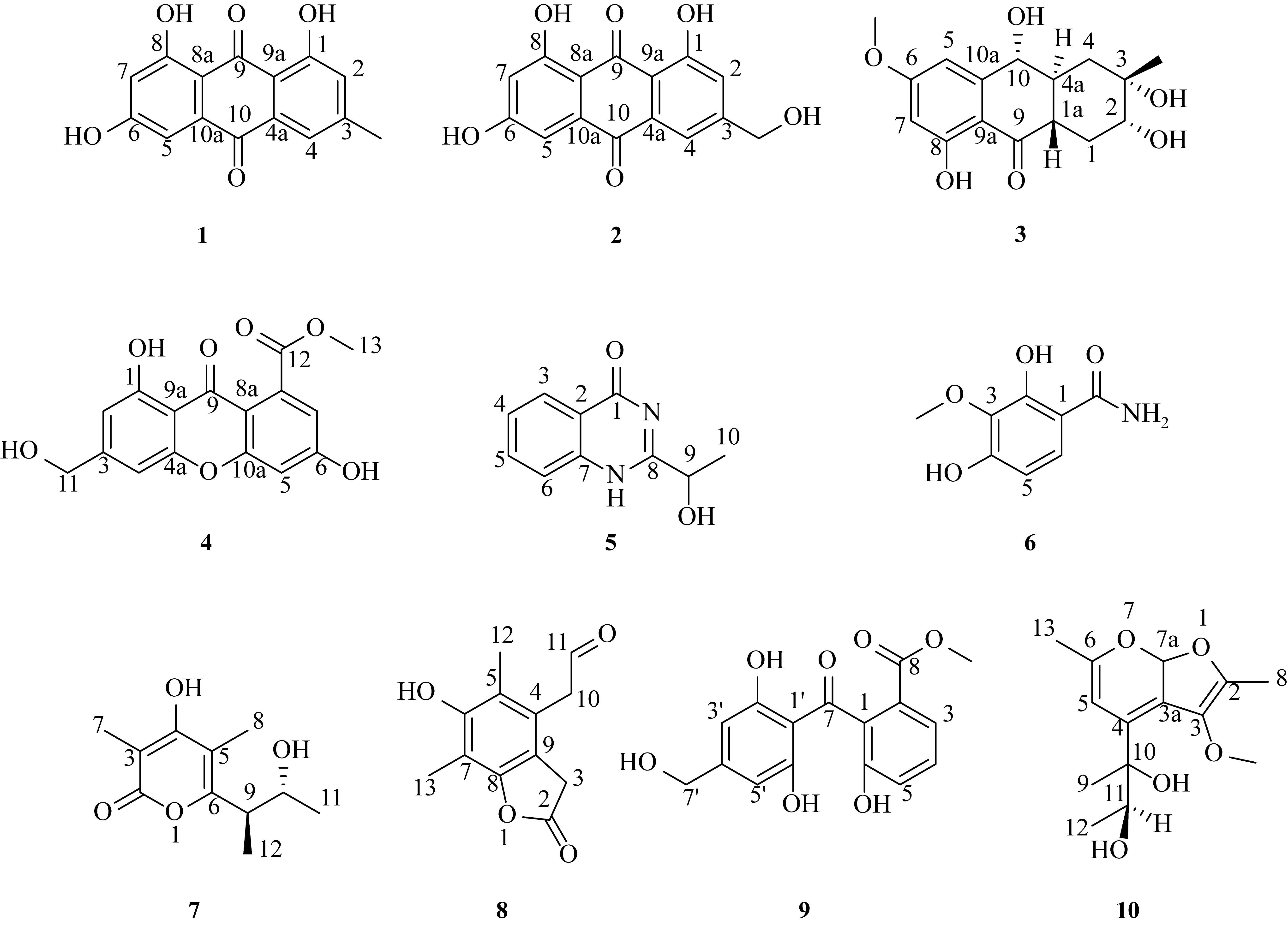
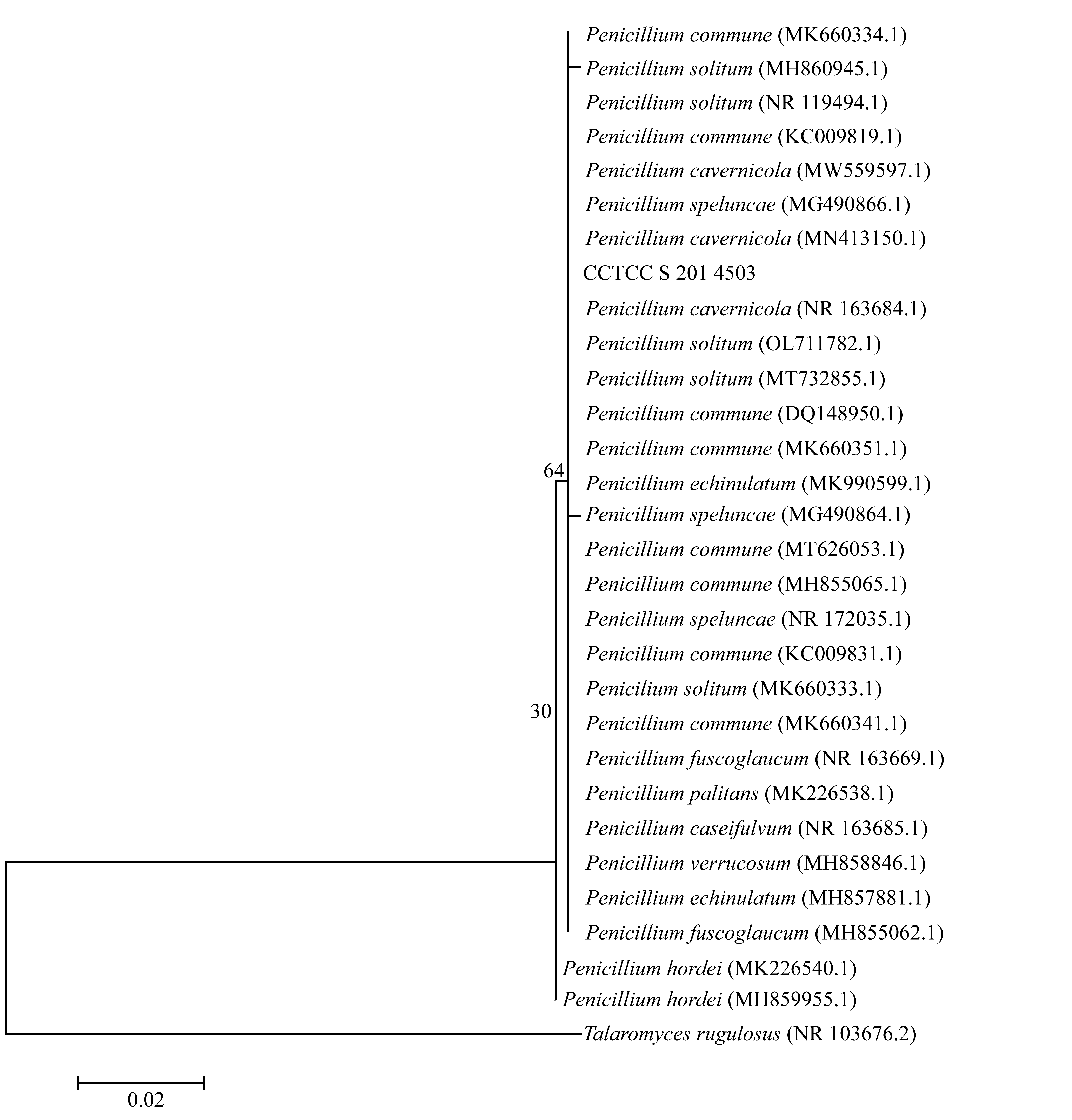
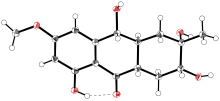
| [1] |
黄筱娟, 陈文豪, 纪明慧, 等, 2015. 菠萝叶的化学成分及生物活性研究[J]. 中草药, 46(7): 949-954.
|
|
HUANG XIAOJUAN, CHEN WENHAO, JI MINGHUI, et al, 2015. Chemical constituents from leaves of ananas comosus and their biological activities[J]. Chinese Traditional and Herbal Drugs, 46(7): 949-954. (in Chinese with English abstract)
|
| [2] |
王淑玲, 孙云廷, 刘昱霞, 等, 2007. 大黄素的药理学研究近况[J]. 中成药, 29(6): 877-879. (in Chinese)
|
| [3] |
徐燕, 田沙沙, 朱华结, 2015. 海洋真菌灰黄青霉Penicillium griseofulvum次级代谢产物中一个新的内酯醛结构[J]. 天然产物研究与开发, 27(4): 559-561.
|
|
XU YAN, TIAN SHASHA, ZHU HUAJIE, 2015. A new lactone aldehyde compound isolated from secondary metabolites of marine fungus Penicillium griseofulvum[J]. Natural Product Research And Development, 27(4): 559-561. (in Chinese with English abstract)
|
| [4] |
杨修伟, 古哲明, 马朝梅, 等, 1998. 从何首乌根中分离的一个新的吲哚衍生物[J]. 中草药, 29(1): 5-11.
|
|
YANG XIUWEI, GU ZHEMING, MA CHAOMEI, et al, 1998. A new indole derivative isolated from the root of tuber fleeceflower (Polygonum multiflorum)[J]. Chinese Traditional and Herbal Drugs, 29(1): 5-11 (in English with Chinese abstract).
|
| [5] |
CARROLL A R, COPP B R, DAVIS R A, et al, 2022. Marine natural products[J]. Natural Product Reports, 37(2): 175-223. DOI: 10.1039/d1np00076d.
doi: 10.1039/d1np00076d
|
| [6] |
CHENG XIA, LIANG XIAO, ZHENG ZHI-HUI, et al, 2020. Penicimeroterpenoids a-c, meroterpenoids with rearrangement skeletons from the marine-derived fungus Penicillium sp. SCSIO 41512[J]. Organic Letters, 22(16): 6330-6333.
doi: 10.1021/acs.orglett.0c02160
|
| [7] |
CIMMINO A, ANDOLFI A, ZONNO M C, et al, 2013. Chenopodolans A-C: phytotoxic furopyrans produced by Phoma chenopodiicola, a fungal pathogen of Chenopodium album[J]. Phytochemistry, 96: 208-213.
doi: 10.1016/j.phytochem.2013.10.007
|
| [8] |
COCKERILL F R, WIKLER M A, BUSH K, et al, 2010. Performance standards for antimicrobial disk susceptibility testing, Twentieth informational supplement[M]. CLSI document M100-S20 (ISBN 1-56238-716-2), Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA.
|
| [9] |
DAYALAN S A J, DARWIN P, PRAKASH S, 2011. Comparative study on production, purification of penicillin by Penicillium chrysogenum isolated from soil and citrus samples[J]. Asian Pacific Journal of Tropical Biomedicine, 1(1): 15-19.
doi: 10.1016/S2221-1691(11)60061-0
|
| [10] |
DOLOMANOV O V, BOURHIS L J, GILDEA R J, et al, 2009. OLEX2: a complete structure solution, refinement and analysis program[J]. Journal of Applied Crystallography, 42(2): 339-341.
doi: 10.1107/S0021889808042726
|
| [11] |
KETTERING M, STERNER O, ANKE T, 2004. Antibiotics in the chemical communication of fungi[J]. Zeitschrift Für Naturforschung C, 59(11-12): 816-823.
doi: 10.1515/znc-2004-11-1209
|
| [12] |
KORNSAKULKARN J, SAEPUA S, KOMWIJIT S, et al, 2014. Bioactive polyketides from the fungus Astrocystis sp. BCC 22166[J]. Tetrahedron, 70(12): 2129-2133.
doi: 10.1016/j.tet.2014.02.004
|
| [13] |
LIBERRA K, LINDEQUIST U, 1995. Marine fungi - a prolific resource of biologically active natural products?[J]. Pharmazie, 50(9): 583-588.
|
| [14] |
MARCHESE P, MAHAJAN N, O'CONNELL E, et al, 2020. A novel high-throughput screening platform identifies itaconate derivatives from marine Penicillium antarcticum as inhibitors of mesenchymal stem cell differentiation[J]. Marine Drugs, 18(4): 192.
doi: 10.3390/md18040192
|
| [15] |
NGAN N T T, QUANG T H, KIM K W, et al, 2017. Anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungal strain Penicillium sp. SF-5629[J]. Archives of Pharmacal Research, 40(3): 328-337.
doi: 10.1007/s12272-017-0890-5
|
| [16] |
OKAMURA N, MIMURA K, HARAGUCHI H, et al, 1996. Altersolanol-related compounds from the culture liquid of Alternaria solani[J]. Phytochemistry, 42(1): 77-80.
doi: 10.1016/0031-9422(95)00861-6
|
| [17] |
PANG XIAOYAN, CAI GUODI, LIN XIUPING, et al, 2019. New alkaloids and polyketides from the marine sponge-derived fungus penicillium sp. SCSIO41015[J]. Marine Drugs, 17(7): 398.
doi: 10.3390/md17070398
|
| [18] |
PITT J L, SAMSON R A, FRISVAD J C, 2000. List of accepted species and their synonyms in the family trichocomaceae[M]. Harwood Academic Publishers:9-46.
|
| [19] |
RAJABI S, RAMAZANI A, HAMIDI M, et al, 2015. Artemia salina as a model organism in toxicity assessment of nanoparticles[J]. DARU Journal of Pharmaceutical Sciences, 23(1): 20.
doi: 10.1186/s40199-015-0105-x
|
| [20] |
SHAABAN K A, SHEPHERD M D, AHMED T A, et al, 2012. Pyramidamycins A-D and 3-hydroxyquinoline-2-carboxamide; cytotoxic benzamides from Streptomyces sp. DGC1[J]. Journal of Antibiotics, 65(12): 615-622.
doi: 10.1038/ja.2012.81
|
| [21] |
SHELDRICK G, 2015a. SHELXT-integrated space-group and crystal-structure determination[J]. Acta Crystallographica Section A Found Adv, 71(1): 3-8.
doi: 10.1107/S2053273314026370
|
| [22] |
SHELDRICK G M, 2015b. Crystal structure refinement with SHELXL[J]. Acta Crystallographica Section C-Structural Chemistry, 71(Pt 1): 3-8.
doi: 10.1107/S2053229614024218
|
| [23] |
TAMURA K, NEI M, KUMAR S, 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method[J]. Proceedings of the National Academy of Sciences of the United States of America, 101(30): 11030-11035.
doi: 10.1073/pnas.0404206101
pmid: 15258291
|
| [24] |
TANG TIAN, YIN LONGWU, YANG JING, et al, 2007. Emodin, an anthraquinone derivative from rheum officinale baill, enhances cutaneous wound healing in rats[J]. European Journal of Pharmacology, 567(3): 177-185.
pmid: 17540366
|
| [25] |
TSANTRIZOS Y S, XU XIAOJING, SAURIOL F, et al, 1994. Novel quinazolinones and enniatins from fusarium lateritium nees[J]. Canadian Journal of Chemistry-Revue Canadienne De Chimie, 72(5): 1415-1415.
doi: 10.1139/v94-177
|
| [26] |
WANG YINCHAO, ZHENG ZHIHUI, LIU SHUCHUN, et al, 2010. Oxepinochromenones, furochromenone, and their putative precursors from the endolichenic fungus Coniochaeta sp.[J]. Journal of Natural Products, 73(5): 920-924.
doi: 10.1021/np100071z
|
| [27] |
WELLS J M, COLE R J, KIRKSEY J W, 1975. Emodin, a toxic metabolite of aspergillus wentii isolated from weevil-damaged chestnuts[J]. Appllied Microbiology, 30(1): 26-28.
|
| [28] |
WU GUANGWEI, MA HONGYAN, ZHU TIANJIAO, et al, 2012. Penilactones A and B, two novel polyketides from Antarctic deep-sea derived fungus Penicillium crustosum PRB-2[J]. Tetrahedron, 68(47): 9745-9749.
doi: 10.1016/j.tet.2012.09.038
|
 ), IMRAN Khan1,2, KUMAR Saurav4, ZHANG Liping1,3, FANG Zhuangjie1,2, ZHANG Xinya1,2, PENG Fang5, ZHANG Changsheng1,2,3
), IMRAN Khan1,2, KUMAR Saurav4, ZHANG Liping1,3, FANG Zhuangjie1,2, ZHANG Xinya1,2, PENG Fang5, ZHANG Changsheng1,2,3