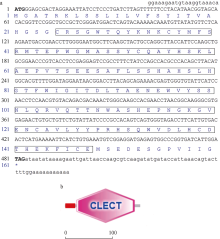
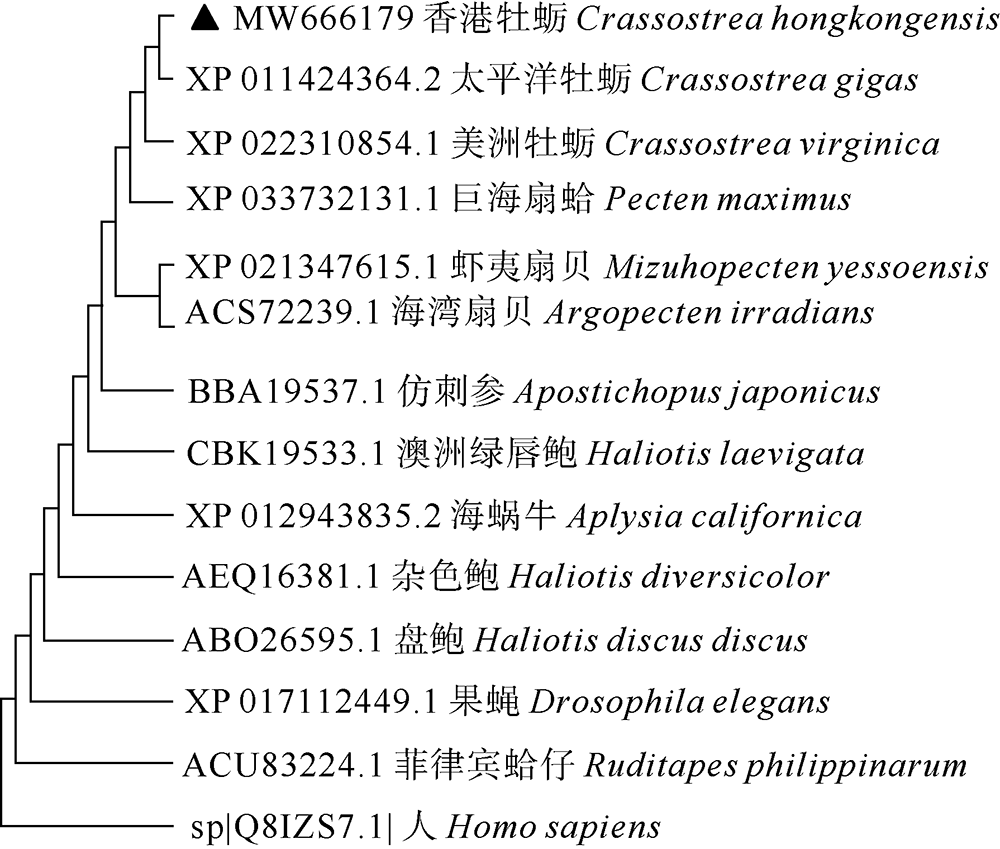
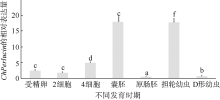
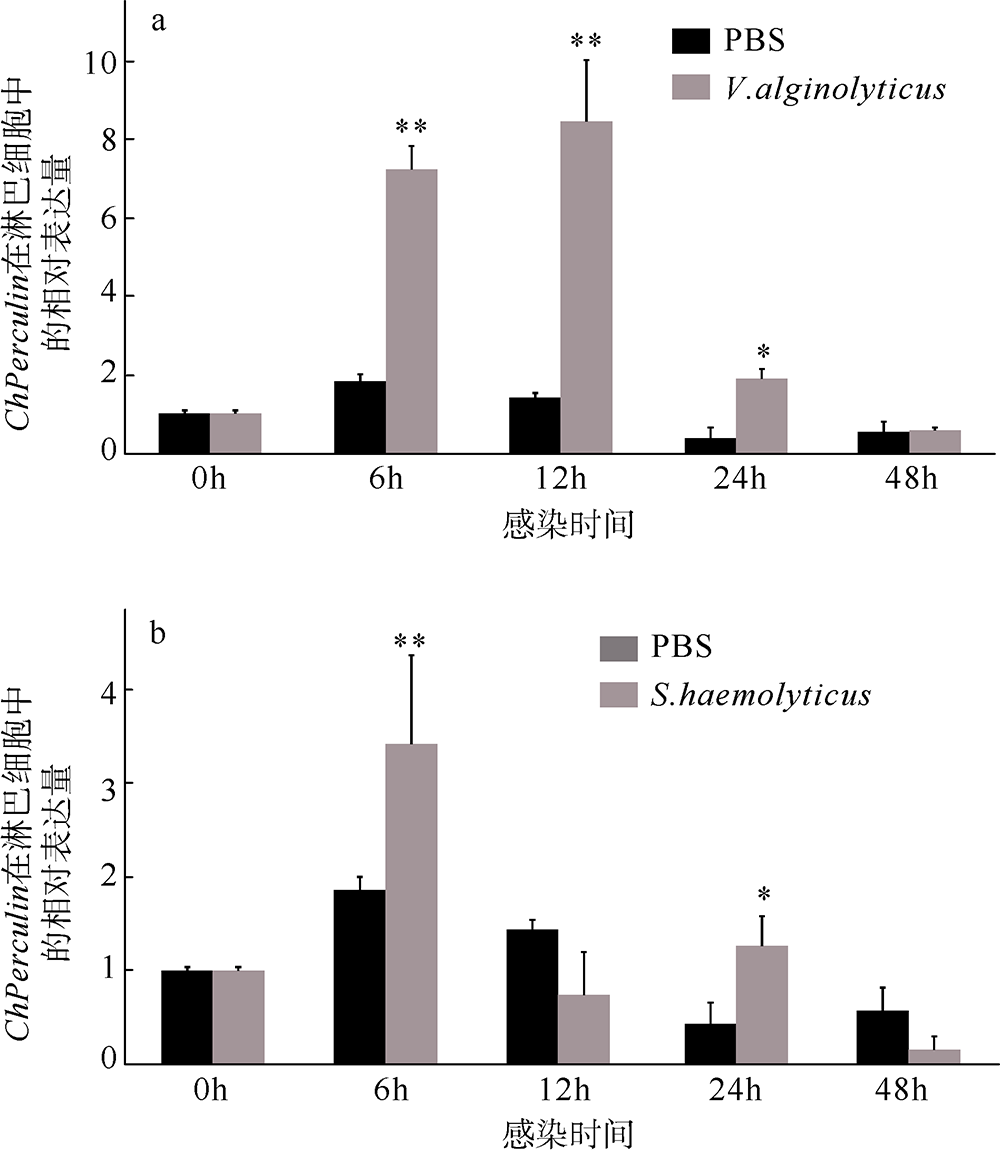
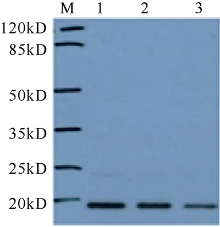
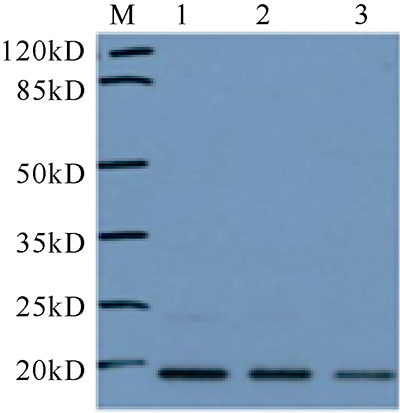
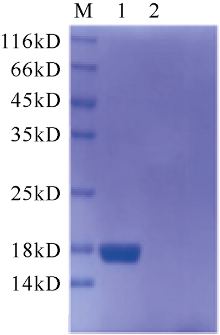
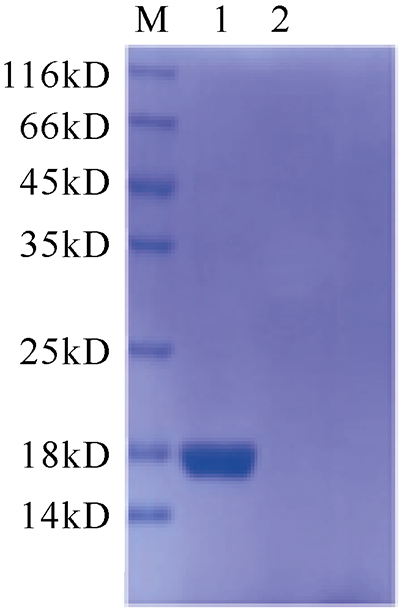
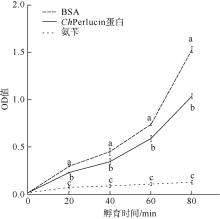
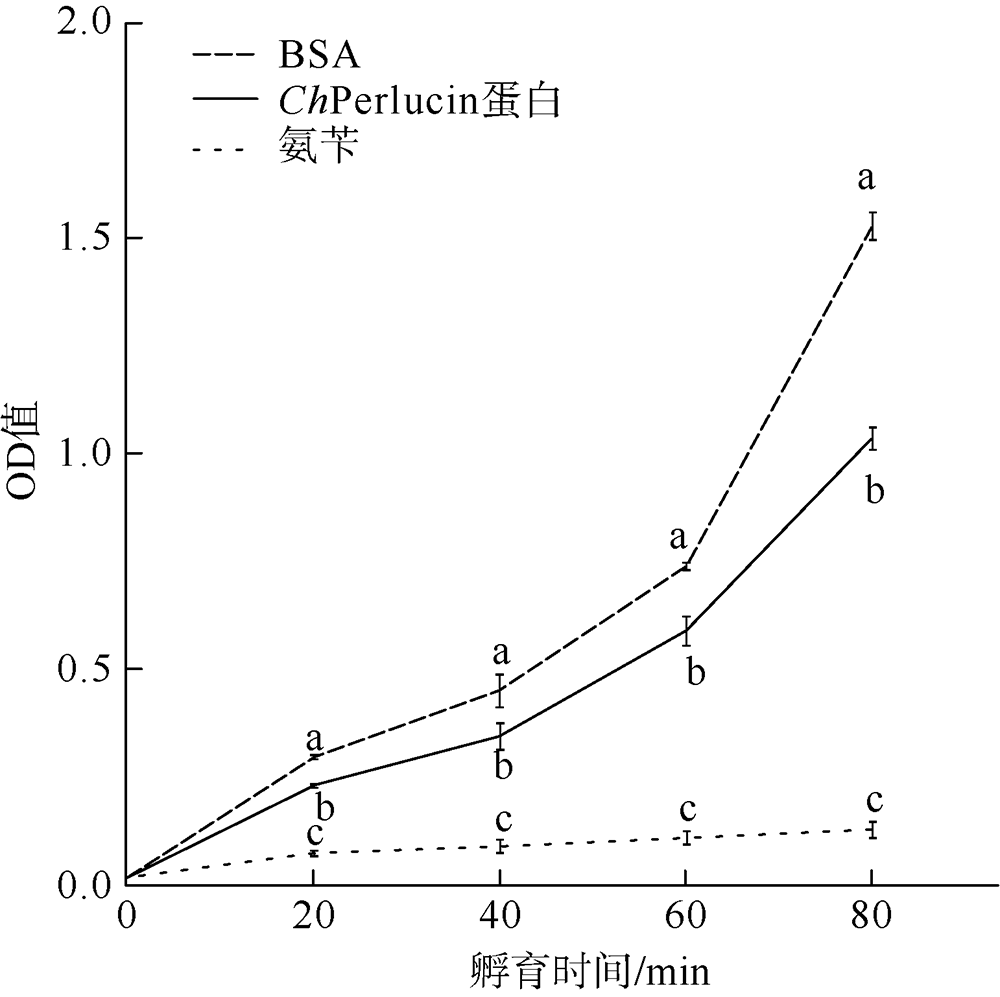
| [1] |
方紫妍, 2016. 中华绒螯蟹C型凝集素(EsLecB)的抗菌功能研究[D]. 上海: 华东师范大学.
|
|
FANG ZIYAN, 2016. The study of a C-type lectin (EsLecB) with antibacterial activity in Eriocheir sinensis[D]. Shanghai: East China Normal University. (in Chinese with English abstract)
|
| [2] |
顾圆圆, 夏正坤, 2008. 甘露糖结合凝集素的研究进展[J]. 医学研究生学报, 21(5): 537-541.
|
|
GU YUANYUAN, XIA ZHENGKUN, 2008. Mannose-binding lectin: from bench to personalized medicine[J]. Journal of Medical Postgraduates, 21(5): 537-541. (in Chinese with English abstract)
|
| [3] |
刘逸尘, 刘丽静, 张亦陈, 等, 2012. 中国明对虾C-型凝集素基因(Fclectin)的重组表达及活性分析[J]. 水产学报, 36(10): 1493-1502.
doi: 10.3724/SP.J.1231.2012.28102
|
|
LIU YICHEN, LIU LIJING, ZHANG YICHEN, et al, 2012. Recombinant expression and functional characterization of a C-type lectin (Fclectin) from the Chinese shrimp (Fenneropenaeus chinensis)[J]. Journal of Fisheries of China, 36(10): 1493-1502. (in Chinese with English abstract)
doi: 10.3724/SP.J.1231.2012.28102
|
| [4] |
农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会, 2019. 中国渔业统计年鉴2019[M]. 北京: 中国农业出版社: 139.
|
|
Fishery Administration of the Ministry of Agriculture and Rural Areas, National Fisheries Technology Extension Center, China Society of Fisheries, 2019. China fishery statisical yearbook[M]. Beijing: China Agriculture Press: 139. (in Chinese)
|
| [5] |
孙盛明, 傅洪拓, 宣富君, 等, 2019. 日本沼虾C型凝集素结构域家族3的cDNA克隆、原核表达和定位分析[J]. 水产学报, 43(11): 2317-2326.
|
|
SUN SHENGMING, FU HONGTUO, XUAN FUJUN, et al, 2019. Molecular cloning, prokaryotic expression and localization analysis of C-type lectin 3 (MnLec3) cDNA from Macrobrachium nipponense[J]. Journal of Fisheries of China, 43(11): 2317-2326. (in Chinese with English abstract)
|
| [6] |
王昊, 2006. 栉孔扇贝凝集素家族基因的研究[D]. 青岛: 中国科学院大学(中国科学院海洋研究所).
|
|
WANG HAO, 2006. Research on lectin family genes of Azumapecten farreri[D]. Qingdao: University of Chinese Academy of Sciences (Institute of Oceanology, (in Chinese with English abstract)
|
| [7] |
BI JINGXIU, NING MINGXIAO, XIE XIAOJUN, et al, 2020. A typical C-type lectin, perlucin-like protein, is involved in the innate immune defense of whiteleg shrimp Litopenaeus vannamei[J]. Fish & Shellfish Immunology, 103: 293-301.
|
| [8] |
DRICKAMER K, 1992. Engineering galactose-binding activity into a C-type mannose-binding protein[J]. Nature, 360(6400): 183-186.
doi: 10.1038/360183a0
|
| [9] |
DRICKAMER K, 1993. Ca2+-dependent carbohydrate-recognition domains in animal proteins[J]. Current Opinion in Structural Biology, 3(3): 393-400.
doi: 10.1016/S0959-440X(05)80112-5
|
| [10] |
ENDO Y, NONAKA M, SAIGA H, et al, 2003. Origin of mannose-binding lectin-associated serine protease (MASP)-1 and MASP-3 involved in the lectin complement pathway traced back to the invertebrate, amphioxus[J]. The Journal of Immunology, 170(9): 4701-4707.
doi: 10.4049/jimmunol.170.9.4701
|
| [11] |
HUANG MENGMENG, SONG XIAOYAN, ZHAO JIANMIN, et al, 2013. A C-type lectin (AiCTL-3) from bay scallop Argopecten irradians with mannose/galactose binding ability to bind various bacteria[J]. Gene, 531(1): 31-38.
doi: 10.1016/j.gene.2013.08.042
|
| [12] |
KANIA P W, SORENSEN R R, KOCH C, et al, 2010. Evolutionary conservation of mannan-binding lectin (MBL) in bony fish: identification, characterization and expression analysis of three bona fide collectin homologues of MBL in the rainbow trout (Onchorhynchus mykiss)[J]. Fish & Shellfish Immunology, 29(6): 910-920.
|
| [13] |
LUO TIAN, YANG HAIJIE, LI FANG, et al, 2006. Purification, characterization and cDNA cloning of a novel lipopolysaccharide-binding lectin from the shrimp Penaeus monodon[J]. Developmental & Comparative Immunology, 30(7): 607-617.
|
| [14] |
MANN K, WEISS I M, ANDRÉ S, et al, 2000. The amino-acid sequence of the abalone (Haliotis laevigata) nacre protein perlucin[J]. European Journal of Biochemistry, 267(16): 5257-5264.
doi: 10.1046/j.1432-1327.2000.01602.x
|
| [15] |
NAKAO M, KAJIYA T, SATO Y, et al, 2006. Lectin pathway of bony fish complement: identification of two homologs of the mannose-binding lectin associated with MASP2 in the common carp (Cyprinus carpio)[J]. The Journal of Immunology, 177(8): 5471-5479.
doi: 10.4049/jimmunol.177.8.5471
|
| [16] |
NETH O, JACK D L, DODDS A W, et al, 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition[J]. Infection and Immunity, 68(2): 688-693.
doi: 10.1128/IAI.68.2.688-693.2000
|
| [17] |
NI Y, TIZARD I, 1996. Lectin-carbohydrate interaction in the immune system[J]. Veterinary Immunology and Immunopathology, 55(1-3): 205-223.
doi: 10.1016/S0165-2427(96)05718-2
|
| [18] |
PRESANIS J S, KOJIMA M, SIM R B, 2003. Biochemistry and genetics of mannan-binding lectin (MBL)[J]. Biochemical Society Transactions, 31(4): 748-752.
doi: 10.1042/bst0310748
|
| [19] |
QU FUFA, XIANG ZHIMING, WANG FUXUAN, et al, 2015. A novel molluscan Fos gene with immune defense function identified in the Hong Kong oyster, Crassostrea hongkongensis[J]. Developmental & Comparative Immunology, 51(1): 194-201.
|
| [20] |
SUN JIE, WANG LEI, WANG BAOJIE, et al, 2008. Purification and characterization of a natural lectin from the plasma of the shrimp Fenneropenaeus chinensis[J]. Fish & Shellfish Immunology, 25(3): 290-297.
|
| [21] |
TAYLOR M E, BRICKELL P M, CRAIG R K, et al, 1989. Structure and evolutionary origin of the gene encoding a human serum mannose-binding protein[J]. The Biochemical Journal, 262(3): 763-771.
doi: 10.1042/bj2620763
|
| [22] |
TIRAPÉ A, BACQUE C, BRIZARD R, et al, 2007. Expression of immune-related genes in the oyster Crassostrea gigas during ontogenesis[J]. Developmental & Comparative Immunology, 31(9): 859-873.
|
| [23] |
WANG LINGLING, YUE FENG, SONG XIAORUI, et al, 2015. Maternal immune transfer in mollusc[J]. Developmental & Comparative Immunology, 48(2): 354-359.
|
| [24] |
WEIS W I, TAYLOR M E, DRICKAMER K, 1998. The C-type lectin superfamily in the immune system[J]. Immunological Reviews, 163(1): 19-34.
doi: 10.1111/imr.1998.163.issue-1
|
| [25] |
YUE FENG, ZHOU ZHI, WANG LINGLING, et al, 2013a. Maternal transfer of immunity in scallop Chlamys farreri and its trans-generational immune protection to offspring against bacterial challenge[J]. Developmental & Comparative Immunology, 41(4): 569-577.
|
| [26] |
YUE FENG, SHI XIAOWEI, ZHOU ZHI, et al, 2013b. The expression of immune-related genes during the ontogenesis of scallop Chlamys farreri and their response to bacterial challenge[J]. Fish & Shellfish Immunology, 34(3): 855-864.
|
| [27] |
ZHANG HAO, PEATMAN E, LIU HONG, et al, 2012. Characterization of a mannose-binding lectin from channel catfish (Ictalurus punctatus)[J]. Research in Veterinary Science, 92(3): 408-413.
doi: 10.1016/j.rvsc.2011.03.024
|
| [28] |
ZHAO ZHIYING, YIN ZHIXIN, XU XIAOPENG, et al, 2009. A novel C-type lectin from the shrimp Litopenaeus vannamei possesses anti-white spot syndrome virus activity[J]. Journal of Virology, 83(1): 347-356.
doi: 10.1128/JVI.00707-08
|
 ), ZHANG Aijiao1,2, YANG Yucheng1,2, MAO Fan1, XIAO Shu1, LI Jun1, ZHANG Yang1, XIANG Zhiming1(
), ZHANG Aijiao1,2, YANG Yucheng1,2, MAO Fan1, XIAO Shu1, LI Jun1, ZHANG Yang1, XIANG Zhiming1( ), YU Ziniu1(
), YU Ziniu1( )
)