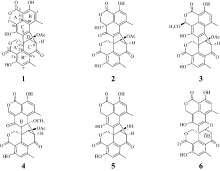
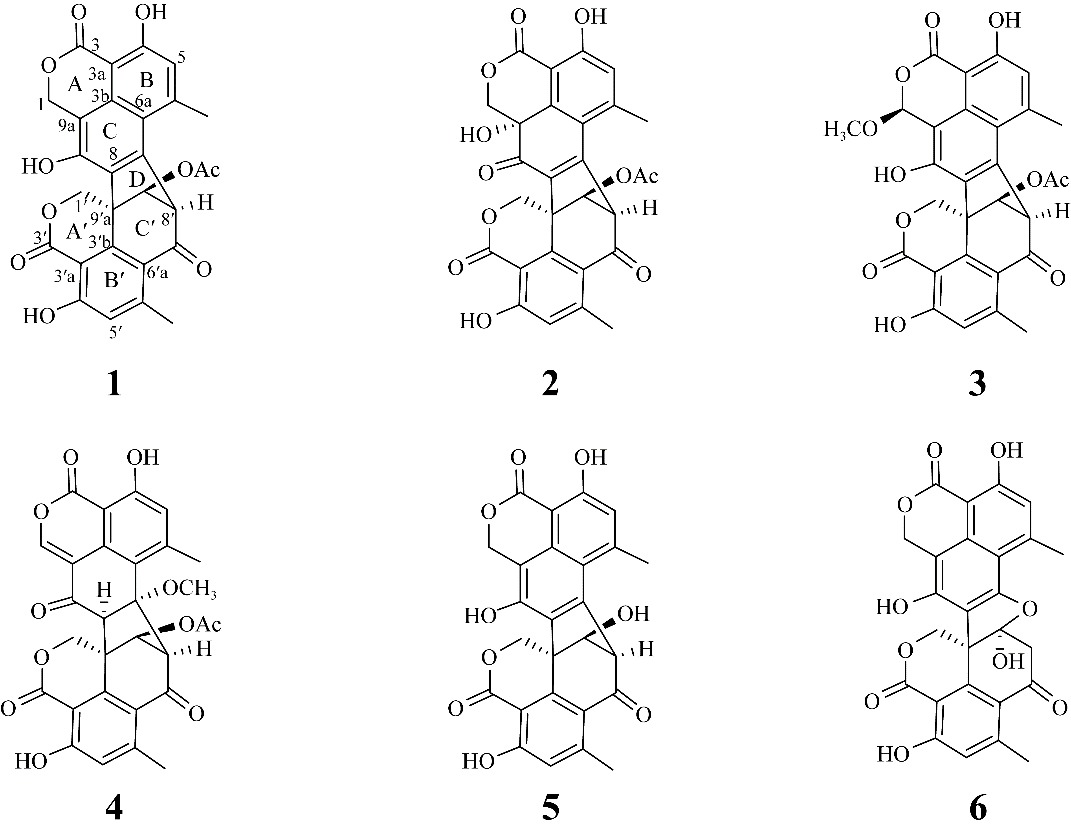
| [1] |
岗云芹, 2020. 白及化学成分及生物活性研究[D]. 武汉: 中南民族大学.
|
|
GANG YUNQIN, 2020. Studies on chemical constituents from Bletilla striata and their biological activities[D]. Wuhan: South-Central University for Nationalities (in Chinese with English abstract).
|
| [2] |
姜薇, 张哲, 单体壮, 等, 2019. 真菌Talaromyces stipitatus WH4-2中的抗菌活性成分[J]. 微生物学报, 59(1): 48-55.
|
|
JIANG WEI, ZHANG ZHE, SHAN TIZHUANG, et al, 2019. Antibacterial compounds from fungus Talaromyces stipitatus WH4-2[J]. Acta Microbiologica Sinica, 59(1): 48-55 (in Chinese with English abstract).
|
| [3] |
马丽丽, 田新朋, 李桂菊, 等, 2021. 海洋微生物来源天然产物研究现状与态势[J]. 热带海洋学报, 40(5): 134-136.
doi: 10.11978/2020104
|
|
MA LILI, TIAN XINPENG, LI GUIJU, et al, 2021. Research status and development trends of natural products from marine microorganisms[J]. Journal of Tropical Oceanography, 40(5): 134-136 (in Chinese with English abstract).
doi: 10.11978/2020104
|
| [4] |
CAO PEI, YANG JING, MIAO CUIPING, et al, 2015. New duclauxamide from Penicillium manginii YIM PH30375 and structure revision of the duclauxin family[J]. Organic Letters, 17(5): 1146-1149.
doi: 10.1021/acs.orglett.5b00081
|
| [5] |
CHAUDHARY N K, CROMBIE A, VUONG D, et al, 2020. Talauxins: Hybrid phenalenone dimers from Talaromyces stipitatus[J]. Journal of Natural Products, 83(4): 1051-1060.
doi: 10.1021/acs.jnatprod.9b01066
|
| [6] |
DENG GAIGAI, WEI WEI, YANG XIUWEI, et al, 2015. New coumarins from the roots of Angelica dahurica var. formosana cv. Chuanbaizhi and their inhibition on NO production in LPS-activated RAW264. 7 cells[J]. Fitoterapia, 101: 194-200.
doi: 10.1016/j.fitote.2015.01.016
|
| [7] |
DETHOUP T, MANOCH L, KIJJOA A, et al, 2006. Bacillisporins D and E, new oxyphenalenone dimers from Talaromyces bacillisporus[J]. Planta Medica, 72(10): 957-960.
doi: 10.1055/s-2006-947188
|
| [8] |
GAO SHUSHAN, DUAN ABING, XU WEI, et al, 2016. Phenalenone polyketide cyclization catalyzed by fungal polyketide synthase and flavin-dependent monooxygenase[J]. Journal of the American Chemical Society, 138(12): 4249-4259.
doi: 10.1021/jacs.6b01528
|
| [9] |
GAO SHUSHAN, ZHANG TAO, GARCIA-BORRAS M, et al, 2018. Biosynthesis of heptacyclic duclauxins requires extensive redox modifications of the phenalenone aromatic polyketide[J]. Journal of the American Chemical Society, 140(22): 6991-6997.
doi: 10.1021/jacs.8b03705
|
| [10] |
NOINART J, BUTTACHON S, DETHOUP T, et al, 2017. A new ergosterol analog, a new Bis-anthraquinone and anti-obesity activity of anthraquinones from the marine sponge-associated fungus Talaromyces stipitatus KUFA 0207[J]. Marine Drugs, 15(5): 139-150.
doi: 10.3390/md15050139
|
| [11] |
SAMARASEKERA K, HUSSEIN W M, WU TAIZONG, et al, 2023. Glyclauxins A-E: dimeric oxaphenalenone aminoglycosides from an Australian wasp nest-derived fungus Talaromyces sp. CMB-MW102[J]. Journal of Natural Products, 86(3): 517-525. doi: 10.1021/acs.jnatprod.2c01069.
|
| [12] |
SHAHID H, CAI TENG, WANG YUYANG, et al, 2021. Duclauxin derivatives from fungi and their biological activities[J]. Front Microbiol, 12: 766440-766459.
doi: 10.3389/fmicb.2021.766440
|
| [13] |
SUN CHUNXIAO, LIU QIANWEN, SHAH M, et al, 2022. Talaverrucin A, heterodimeric oxaphenalenone from Antarctica sponge-derived fungus Talaromyces sp. HDN151403, inhibits Wnt/β-Catenin signaling pathway[J]. Organic Letters, 24(22): 3993-3997.
doi: 10.1021/acs.orglett.2c01394
|
| [14] |
ZANG YI, GENTA-JOUVE G, ESCARGUEIL A E, et al, 2016a. Antimicrobial oligophenalenone dimers from the soil fungus Talaromyces stipitatus[J]. Journal of Natural Products, 79(12): 2991-2996.
doi: 10.1021/acs.jnatprod.6b00458
|
| [15] |
ZANG YI, GENTA-JOUVE G, RETAILLEAU P, et al, 2016b. Talaroketals A and B, unusual bis(oxaphenalenone) spiro and fused ketals from the soil fungus Talaromyces stipitatus ATCC 10500[J]. Organic & Biomolecular Chemistry, 14(9): 2691-2697.
|
| [16] |
ZANG YI, GENTA-JOUVE G, SUN T A, et al, 2015. Unexpected talaroenamine derivatives and an undescribed polyester from the fungus Talaromyces stipitatus ATCC10500[J]. Phytochemistry, 119: 70-75.
doi: 10.1016/j.phytochem.2015.09.002
|
| [17] |
ZHANG MI, DENG YANFANG, LIU FEI, et al, 2021. Five undescribed steroids from Talaromyces stipitatus and their cytotoxic activities against hepatoma cell lines[J]. Phytochemistry, 189: 112816-112825.
doi: 10.1016/j.phytochem.2021.112816
|
| [18] |
ZHANG MI, YAN SHAN, LIANG YU, et al, 2020. Talaronoids A-D: Four fusicoccane diterpenoids with an unprecedented tricyclic 5/8/6 ring system from the fungus Talaromyces stipitatus[J]. Organic Chemistry Frontiers, 7(21): 3486-3492.
doi: 10.1039/D0QO00960A
|
 ), PAN Chao, HUA Silu, JIANG Wei(
), PAN Chao, HUA Silu, JIANG Wei( )
)