| [1] |
BISTER B, BISCHOFF D, STRÖBELE M, et al, 2004. Abyssomicin c-a polycyclic antibiotic from a marine Verrucosispora strain as an inhibitor of the p-aminobenzoic acid/tetrahydrofolate biosynthesis pathway[J]. Angewandte Chemie International Edition, 43(19): 2574-2576.
doi: 10.1002/(ISSN)1521-3773
|
| [2] |
CLSI, 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grows aerobically. 9th ed.[M]. CLSI document m07-a9. Wayne, PA: Clinical and laboratory standards institute.
|
| [3] |
FREUNDLICH J S, LALGONDAR M, WEI JUN-RONG, et al, 2010. The abyssomicin C family as in vitro inhibitors of Mycobacterium tuberculosis[J]. Tuberculosis (Edinb), 90(5): 298-300.
doi: 10.1016/j.tube.2010.08.002
|
| [4] |
HUANG HONGBO, SONG YONGXIANG, LI XIN, et al, 2018. Abyssomicin monomers and dimers from the marine-derived Streptomyces koyangensis SCSIO 5802[J]. Journal of Natural Products, 81(8): 1892-1898.
doi: 10.1021/acs.jnatprod.8b00448
|
| [5] |
JI XIAOQI, TU JIAJIA, SONG YONGXIANG, et al, 2020. A luciferase-like monooxygenase and flavin reductase pair abme2/abmz catalyzes baeyer-villiger oxidation in neoabyssomicin biosynthesis[J]. ACS Catalysis, 10(4): 2591-2595.
doi: 10.1021/acscatal.9b05488
|
| [6] |
LEÓN B, NAVARRO G, DICKEY B J, et al, 2015. Abyssomicin 2 reactivates latent hiv-1 by a pkc- and hdac-independent mechanism[J]. Organic Letters, 17(2): 262-265.
doi: 10.1021/ol503349y
pmid: 25560385
|
| [7] |
LI QINGLIAN, DING WENJUAN, YAO ZIWEI, et al, 2018. Abmv catalyzes tandem ether installation and hydroxylation during neoabyssomicin/abyssomicin biosynthesis[J]. Organic Letters, 20(16): 4854-4857.
doi: 10.1021/acs.orglett.8b01997
pmid: 30070849
|
| [8] |
RIEDLINGER J, REICKE A, ZÄHNER H, et al, 2004. Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine Verrucosispora strain ab-18-032[J]. Journal of Antibiotics, 57(4): 271-279.
doi: 10.7164/antibiotics.57.271
|
| [9] |
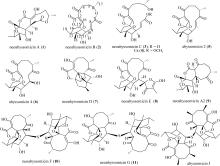
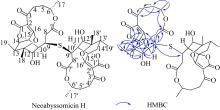
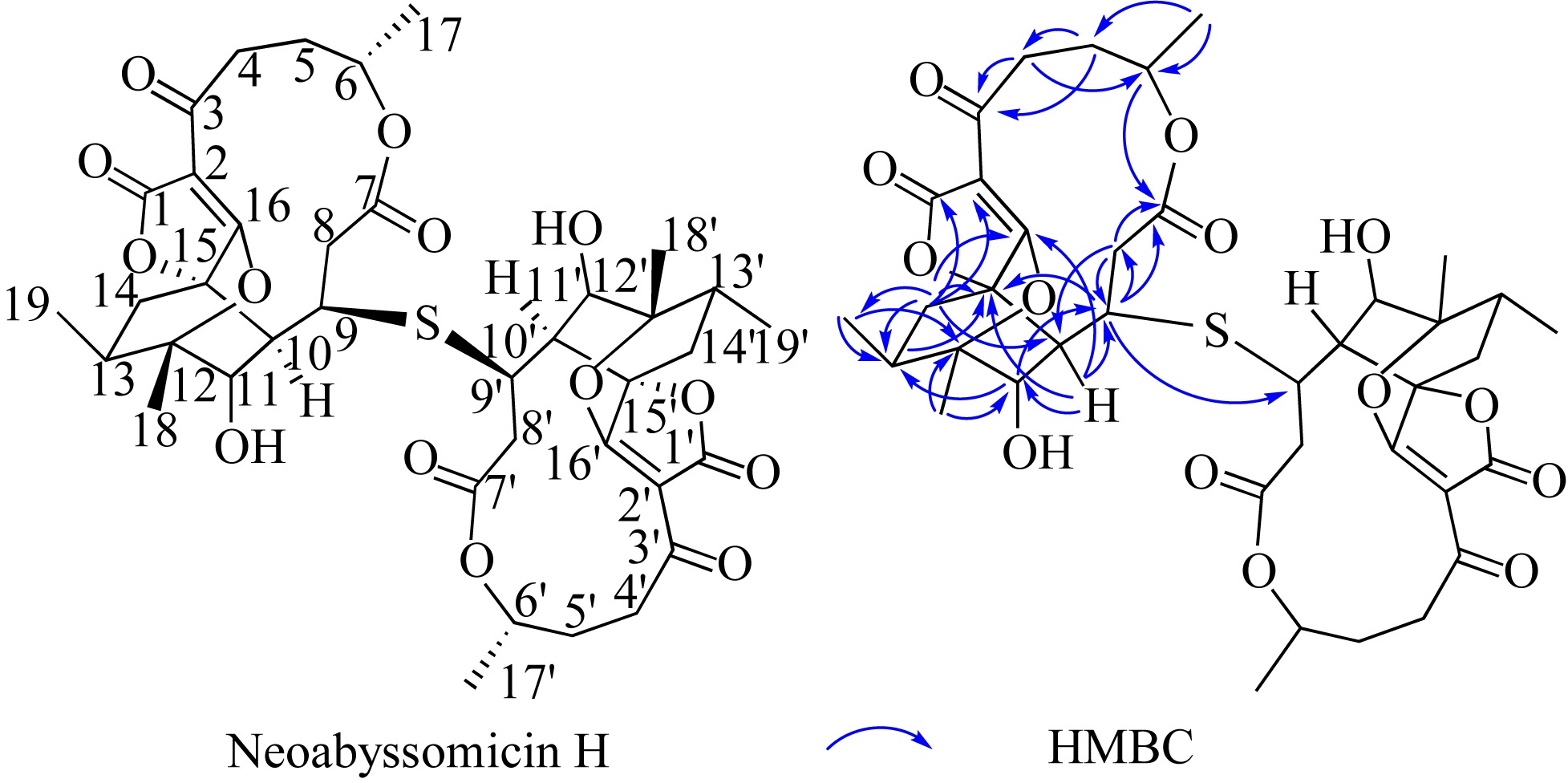
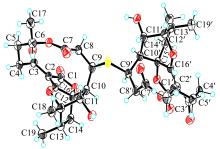
SONG YONGXIANG, LI QINGLIAN, QIN FENGXIANG, et al, 2017. Neoabyssomicins a-c, polycyclic macrolactones from the deep-sea derived Streptomyces koyangensis SCSIO 5802[J]. Tetrahedron, 73(36): 5366-5372.
doi: 10.1016/j.tet.2017.07.034
|
| [10] |
TU JIA, LI SITING, CHEN JIANG, et al, 2018. Characterization and heterologous expression of the neoabyssomicin/ abyssomicin biosynthetic gene cluster from Streptomyces koyangensis SCSIO 5802[J]. Microbial Cell Factories, 17(1): 28.
doi: 10.1186/s12934-018-0875-1
|
 ), LI Xiaoyue1,3, JU Jianhua1,2,3,4
), LI Xiaoyue1,3, JU Jianhua1,2,3,4