Journal of Tropical Oceanography ›› 2024, Vol. 43 ›› Issue (1): 94-106.doi: 10.11978/2023040CSTR: 32234.14.2023040
• Marine Biology • Previous Articles Next Articles
Expression characteristics and function of Anti-Müllerian hormone receptor Ⅱ gene (amhr2) in Paralichthys olivaceus
LI Ze1,2,3( ), WANG Lijuan1,2(
), WANG Lijuan1,2( ), ZOU Congcong1,2,3, SHU Chang1,2,3, WU Zhihao1,2, ZOU Yuxia1,2, YOU Feng1,2(
), ZOU Congcong1,2,3, SHU Chang1,2,3, WU Zhihao1,2, ZOU Yuxia1,2, YOU Feng1,2( )
)
- 1. Chinese Academy of Sciences and Shandong Province Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science (Institute of Oceanology, Chinese Academy of Sciences), Qingdao 266071, China
2. Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
3. University of Chinese Academy of Sciences, Beijing 100049, China
-
Received:2023-03-24Revised:2023-04-21Online:2024-01-10Published:2024-01-19 -
Supported by:National Key R&D Program of China(2022YFD2400402); National Key R&D Program of China(2018YFD0900202); Shandong Natural Science Foundation(ZR2022MC026); Youth Research Fund of Marine Biology and Biotechnology Laboratory, Pilot National Laboratory for Marine Science and Technology (Qingdao)(YQ2018NO01)
Cite this article
LI Ze, WANG Lijuan, ZOU Congcong, SHU Chang, WU Zhihao, ZOU Yuxia, YOU Feng. Expression characteristics and function of Anti-Müllerian hormone receptor Ⅱ gene (amhr2) in Paralichthys olivaceus[J].Journal of Tropical Oceanography, 2024, 43(1): 94-106.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Tab. 1
Primers used in this study"
| 引物 | 序列 | NCBI序列号及参考文献 |
|---|---|---|
| amhr2-CDS-F | ATTCCCAGGACCCTCCCTGTT | XM_020096590.1 |
| amhr2-CDS-R | TCTGCAAAGAGAACAGTGCAAC | |
| amh-CDS-F | ATGCCGGTGGTGAACGTCTT | XM_020080898.1 |
| amh-CDS-R | GCGGCATCCACACTCCTTTG | |
| amhr2-qPCR-F | CGTCGTGTTCCTGCCAAAC | XM_020096590.1 |
| amhr2-qPCR-R | GGCAGCTCATACACCTCCTT | |
| actin- qPCR-F | GGAATCCACGAGACCACCTACA | EU090804 Hu et al, |
| actin- qPCR-R | CTGCTTGCTGATCCACATCTGC | |
| ef- qPCR-F | AGCCAGAAGCGTTTTGAGGAG | XM_020104638.1 Zhong et al, |
| ef- qPCR-R | AGATGGGGACGAAAGCAACAC | |
| amh-pc3.1-EcoRV-F | CACACTGGACTAGTGGATCCGCCACCATGCCGGTGGTGAACGTC | XM_020080898.1 |
| amh-pc3.1-EcoRV-R | AGCTTGGTACCGAGCTCGGATCCGCGGCATCCACACTCCT | |
| amhr2-pc3.1-BamHI-F | CACACTGGACTAGTGGATCCGCCACCATGATGCTGCAGTGGTGGC | XM_020096590.1 |
| amhr2-pc3.1-BamHI-R | AGCTTGGTACCGAGCTCGGATCCTGAGCTGCTTTCAGAGACATAAGACTG | |
| amhr2-pCS2-BamHI-F | ACGACGACGATAAGGGATCCGCCACCATGATGCTGCAGTGGTGGC | XM_020096590.1 |
| amhr2-pCS2-BamHI-R | AATTCGAATCGATGGGATCCTGAGCTGCTTTCAGAGACATAAGACTG | |
| amh-pET-EcoRV-F | AAGGCCATGGCTGATATGCCGGTGGTGAACGTCTT | XM_020080898.1 |
| amh-pET-EcoRV-R | GAATTCGGATCCGATGCGGCATCCACACTCCTTTG |

Fig. 1
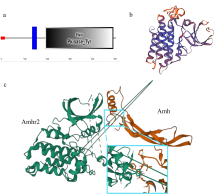
Amino acid sequence alignment and secondary structure prediction of Amhr2 among Paralichthys olivaceus and other species. P. olivaceus is marked with star. α, α- helices structure; β, β- sheets structure; black area, the sequence completely conserved region; dotted line box, the GL ×× LH conservative motif; full line box, the AH × D ×××× N × L conservative motif"

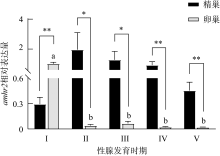
Fig. 5
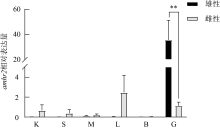
The expression of amhr2 in P. olivaceus during the gonadal development. n=3. Different letters indicate the difference in the ovaries of the fish at different gonadal development stages. * indicates the difference between the testes and ovaries of the fish at the same gonadal development stages, P< 0.05; **, P< 0.01"
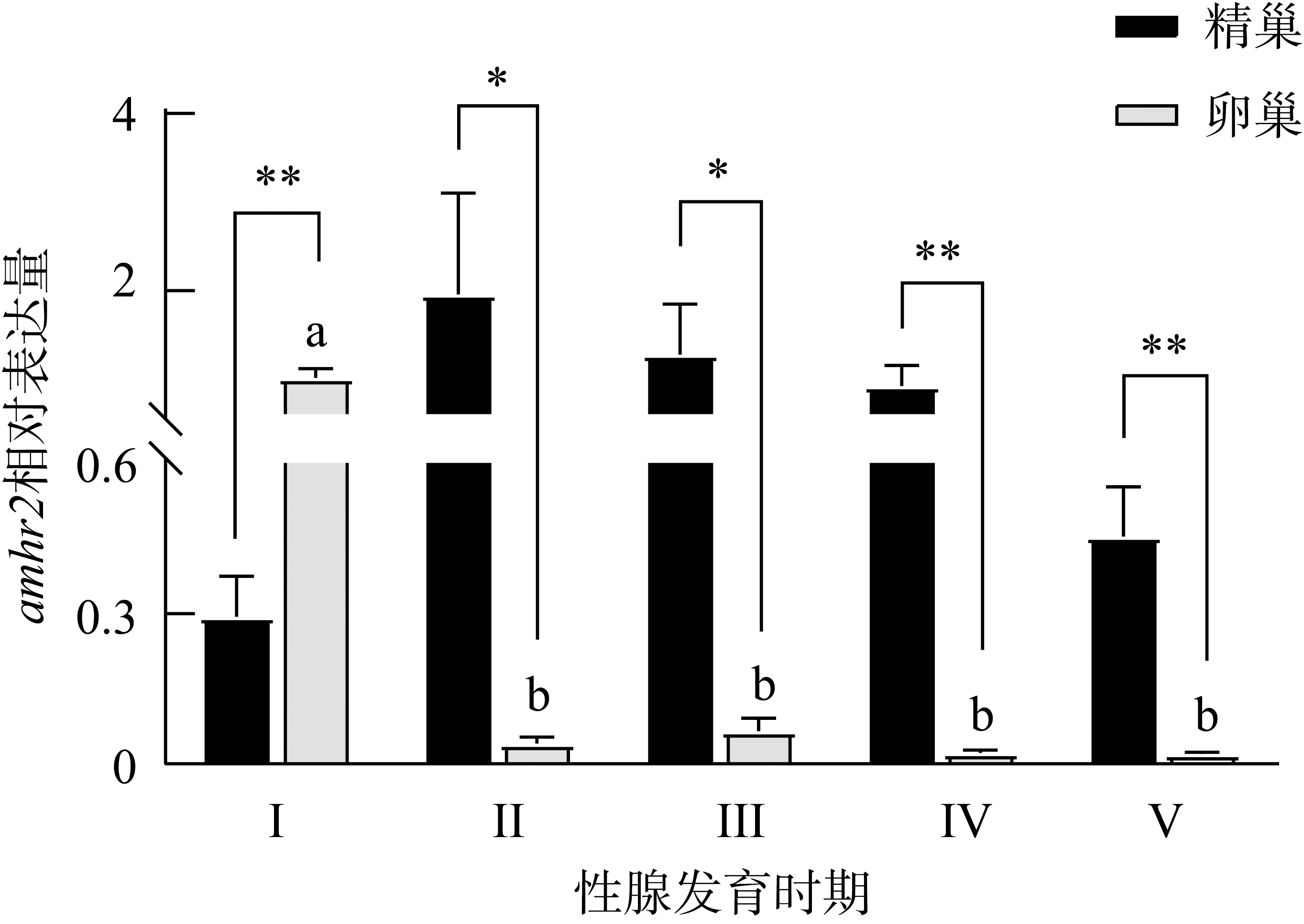
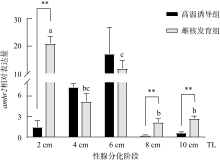
Fig. 6
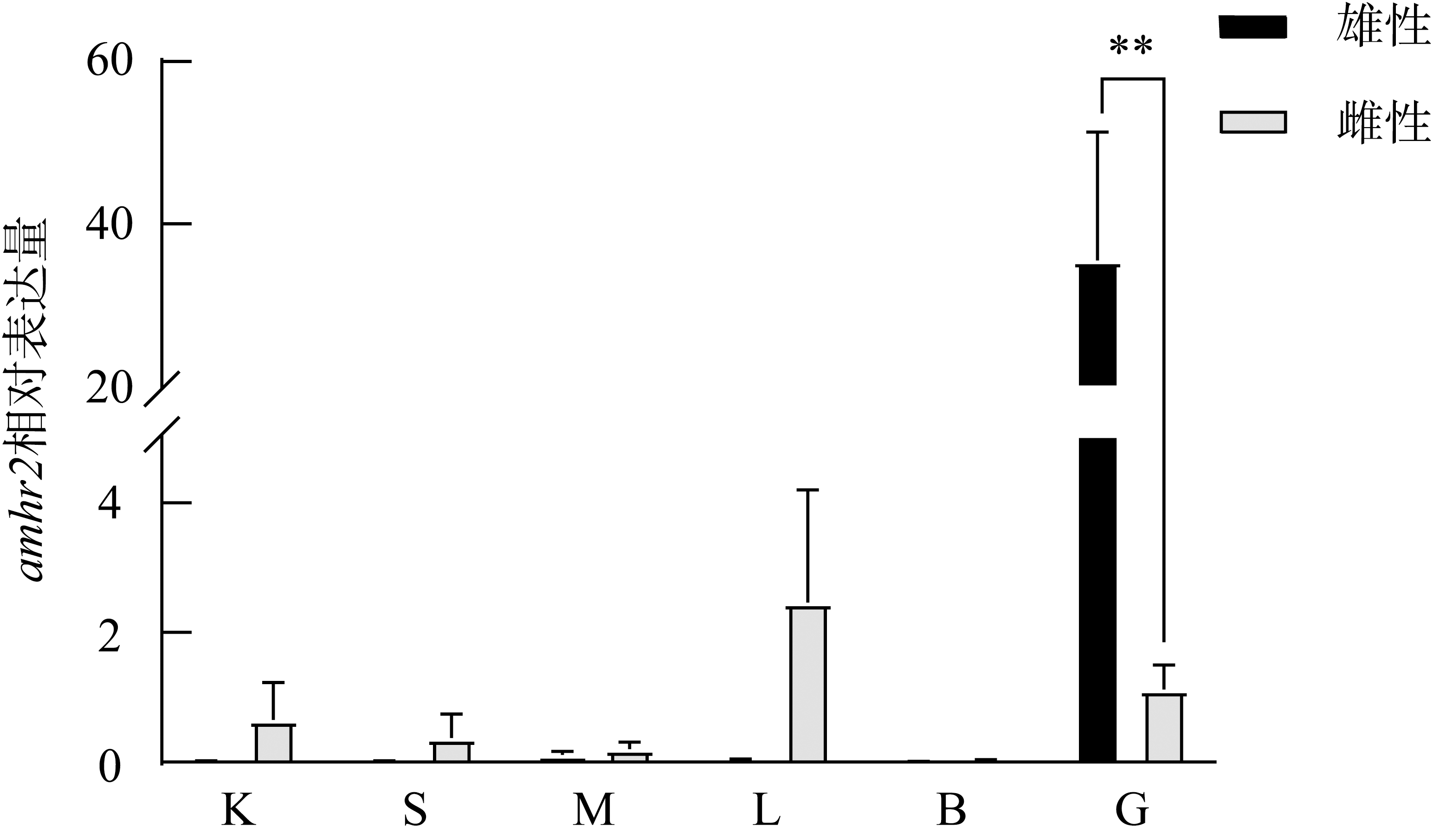
The expression of amhr2 in P. olivaceus during gonadal differentiation. n=3. Different letters indicate the differences at different TLs of the gynogenesis control group during the gonadal differentiation, P< 0.05. ** indicates the difference between the testes and ovaries of the fish at the same TLs, P< 0.01"
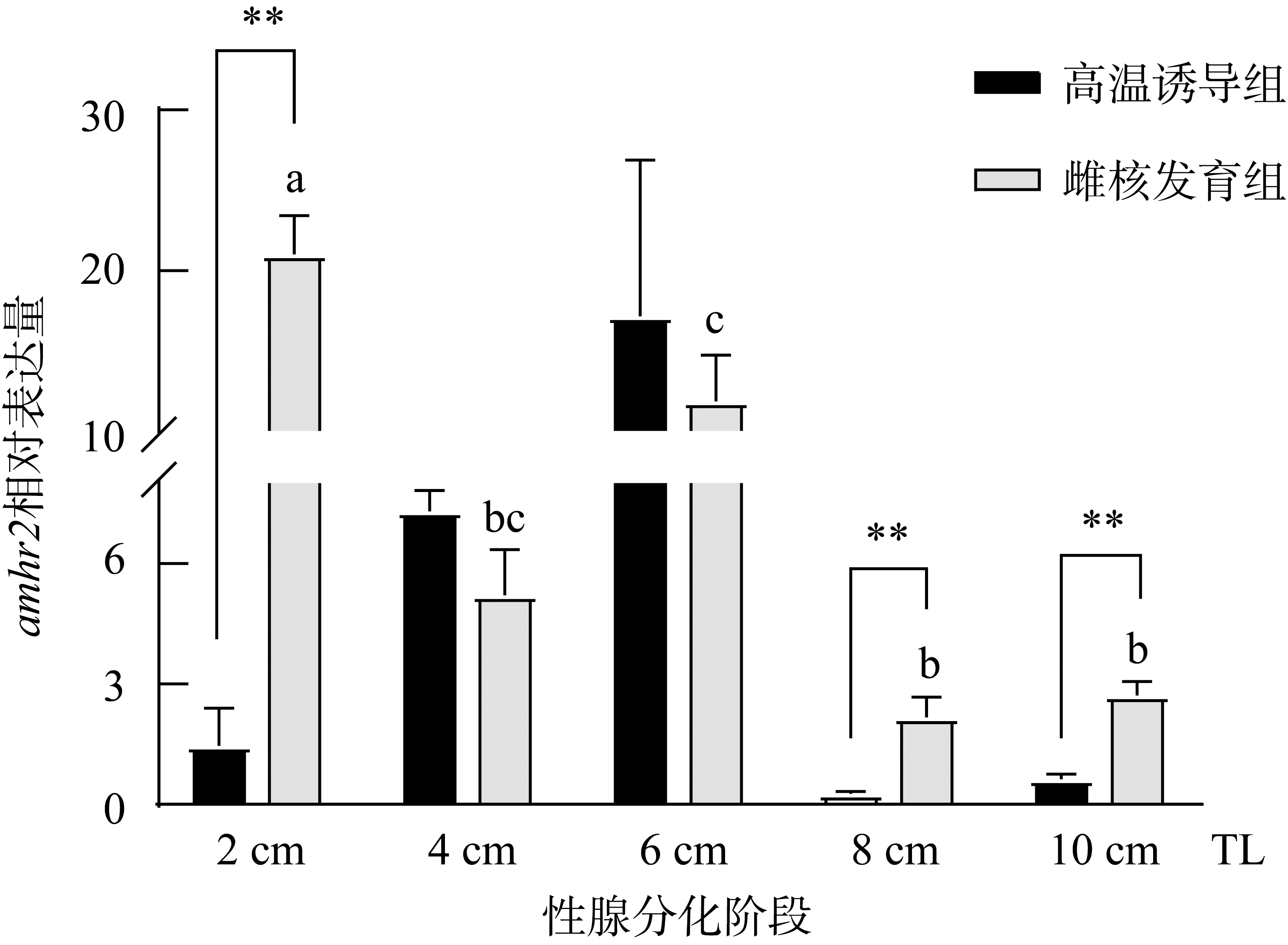
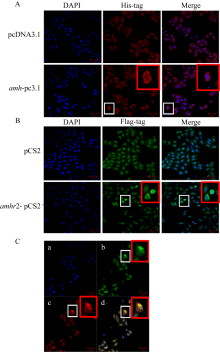
Fig. 7
Subcellular localization of Amh and Amhr2 of P. olivaceus. (a) Amh, pcDNA3.1, blank vector plasmid; amh-pc3.1, recombinant plasmid of amh; (B) Amhr2, pCS2, blank vector plasmid; amhr2-pCS2, recombinant plasmid of amhr2; (c) the co-localization of Amh and Amhr2 (a. DAPI; b. anti-Flag; c. anti-His; d. anti-Flag+ anti-His). Red box, the enlarged image of the white box, bar=50 μm"

Fig.8
Expression and purification of the recombinant P. olivaceus Amh. (a) SDS-PAGE results of Amh prokaryotic expression (M, marker; 1, blank medium; 2, experimental medium; 3, control thallus precipitation; 4, Amh thallus precipitation; 5, blank cell supernatant; 6, Amh cell supernatant); (b) SDS-PAGE results of recombinant Amh purification (M, marker; 1-7, protein elution in each step); (c) WB results of Amh prokaryotic expression (M, marker; 1, blank medium; 2, Amh thallus precipitation; 3, supernatant after the bacterial fragmentation; 4, precipitation after the bacterial fragmentation. Box, the recombinant Amh)"

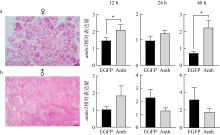
Fig. 9
The changes of amhr2 expression in the ovary and testis incubated with the recombinant Amh. (a) Histological section of the ovary and the expression of amhr2 after 12, 24, and 48 h incubation with recombinant Amh in the ovaries; (b) histological observation of the testis and the expression of amhr2 after 12, 24, 48 h incubation with the recombinant Amh in the testes. n=4. bar=50μm. * indicates the difference between different groups, P< 0.05"

| [1] |
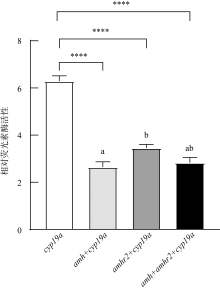
范兆飞, 2017. 牙鲆cyp19a及其转录因子表观修饰和调控研究[D]. 青岛: 中国科学院大学: 中国科学院海洋研究所: 1-137.
|
|
|
|
| [2] |
高莹莹, 胡鹏, 刘新富, 等, 2019. 暗纹东方鲀抗苗勒氏管激素Ⅱ型受体基因的克隆、生物信息学及表达分析[J]. 海洋渔业, 41(5): 555-566.
|
|
|
|
| [3] |
韩玉龙, 2019. amh基因在斜带石斑鱼性别分化中的作用机制研究[D]. 广州: 中山大学: 1-130.
|
|
|
|
| [4] |
孙鹏, 尤锋, 张立敬, 等, 2009. 牙鲆性腺分化的组织学研究[J]. 海洋科学, 33(3): 53-58.
|
|
|
|
| [5] |
王妹, 邓思平, 陈华谱, 等, 2018. 金钱鱼Amhr2基因的克隆及表达分析[J]. 广东海洋大学学报, 38(3): 17-24.
|
|
|
|
| [6] |
赵九娥, 2015. Amhy/Amh及其受体AmhrⅡ对尼罗罗非鱼雄性性别的决定作用[D]. 重庆: 西南大学: 1-80.
|
|
|
|
| [7] |
doi: 10.1038/ncomms10055 pmid: 26753790 |
| [8] |
doi: 10.1007/s11033-021-06606-4 |
| [9] |
|
| [10] |
doi: 10.1016/j.aquaculture.2018.03.008 |
| [11] |
doi: 10.3389/fendo.2019.00210 |
| [12] |
|
| [13] |
doi: 10.3389/fgene.2022.1007548 |
| [14] |
doi: 10.1073/pnas.1018392109 pmid: 22323585 |
| [15] |
|
| [16] |
doi: 10.1371/journal.pone.0108582 |
| [17] |
|
| [18] |
doi: 10.1371/journal.pgen.1002798 |
| [19] |
doi: 10.1002/dvdy.v236:1 |
| [20] |
|
| [21] |
doi: 10.1371/journal.pgen.1005678 |
| [22] |
doi: 10.1677/jme.1.01853 |
| [23] |
|
| [24] |
doi: 10.1098/rstb.2020.0091 |
| [25] |
doi: 10.1016/j.ygcen.2015.09.025 |
| [26] |
doi: 10.1016/j.modgep.2005.02.008 |
| [27] |
doi: 10.1371/journal.pone.0081551 |
| [28] |
|
| [29] |
doi: 10.1007/s11802-022-4898-1 |
| [30] |
doi: 10.1016/j.ijbiomac.2022.06.098 |
| [31] |
doi: 10.1007/s00427-015-0495-2 |
| [32] |
doi: 10.1016/j.aquaculture.2022.738984 |
| [33] |
doi: 10.1016/j.gene.2020.144906 |
| [34] |
|
| [35] |
doi: 10.3390/ijms24032480 |
| [36] |
doi: 10.1016/j.cub.2012.05.045 |
| [37] |
doi: 10.1016/j.bbrc.2004.07.162 |
| [38] |
doi: 10.1007/BF02863039 |
| [39] |
doi: 10.3390/fishes7030129 |
| [40] |
|
| [41] |
|
| [42] |
doi: 10.1007/s10126-007-9064-7 |
| [1] | RAO Yiyong, ZHAO Meirong, KUANG Zexing, HUANG Honghui, TAN Erhui. Influence of raft-string oyster culture on the functional structure of macrobenthic communities: a case study in the Dapeng Cove* [J]. Journal of Tropical Oceanography, 2024, 43(5): 69-83. |
| [2] | YUAN Xiangcheng, LIANG Yuxian, SONG Yan, YU Xiaolei, HUANG Hui, ZHOU Weihua. Effects of ocean acidification on the calcification and gene expression in coral Acropora hyacinthus* [J]. Journal of Tropical Oceanography, 2024, 43(3): 40-48. |
| [3] | JIA Nan, ZHOU Tiancheng, HU Simin, ZHANG Chen, HUANG Hui, LIU Sheng. Difference in the feeding contents of three hermit crabs in the coral reefs of the Nansha Islands, South China Sea [J]. Journal of Tropical Oceanography, 2024, 43(3): 109-121. |
| [4] | LI Cai, LIU Cong, ZHANG Xianqing, CHEN Fei, XIAO Zhihui, YANG Zeming, ZHENG Yuanning, ZHOU Wen, XU Zhantang. Development and Application of the Multiangle Volume Scattering and Attenuation Meter (VSAM)* [J]. Journal of Tropical Oceanography, 2024, 43(2): 1-11. |
| [5] | SUN Tingting, HAO Wenjin, XU Pengzhen, YE Lijing, DONG Zhijun. Effects of seawater acidification on microorganisms associated with Aurelia coerulea polyps [J]. Journal of Tropical Oceanography, 2023, 42(6): 111-119. |
| [6] | ZHANG Xianqing, LI Cai, Zhou Wen, LIU Cong, XU Zhantang, CAO Wenxi, YANG Yuezhong. Studying on diffuse attenuation coefficient in the South China Sea based on volume scattering function and absorption coefficient* [J]. Journal of Tropical Oceanography, 2023, 42(3): 86-95. |
| [7] | TANG Chaoli, TAO Xinhua, WEI Yuanyuan, DAI Congming, WEI Heli. Spatiotemporal modal analysis and prediction of surface temperature in East Asia and the Western Pacific* [J]. Journal of Tropical Oceanography, 2022, 41(6): 183-192. |
| [8] | XU Hanzhi, ZHANG Hua, XIONG Panpan, HE Maoxian. Differential responses of allometric individuals to immune stimuli in Pinctada fucata martensii [J]. Journal of Tropical Oceanography, 2022, 41(5): 180-188. |
| [9] | FENG Bingbin, WANG Riming, LI Shushi, HUANG Hu, HU Baoqing. Changes of the artificial beach profile in the Qinzhou Bay [J]. Journal of Tropical Oceanography, 2022, 41(4): 51-60. |
| [10] | CHEN Kehai, XIE Xuetong, ZHANG Jinlan, ZHENG Yan. An SST dependent geophysical model function for HY-2A scatterometer [J]. Journal of Tropical Oceanography, 2022, 41(2): 90-102. |
| [11] | LI Ao, FENG Yang, WANG Yuntao, XUE Huijie. Spatiotemporal variation of water area with high chlorophyll a concentration in the South China Sea based on OC-CCI data* [J]. Journal of Tropical Oceanography, 2022, 41(2): 77-89. |
| [12] | PAN Xiaolan, LIU Huiru, XU Meng, XU Hanzhi, ZHANG Hua, HE Maoxian. Preliminary study on immunity function of aquaporin AQP4 in the pearl oyster Pinctada fucata martensii [J]. Journal of Tropical Oceanography, 2021, 40(2): 83-89. |
| [13] | Jiahao NI, Xiaojing ZHU, Yiping JI, Bin ZHOU, Yajun WANG, Shanliang XU, Danli WANG. Effects of breeding density on the growth, metabolic enzyme activity and related gene expression level of juvenile Pampus argenteus [J]. Journal of Tropical Oceanography, 2020, 39(2): 54-64. |
| [14] | Shibing ZHU,Danni HU,Huiling ZHANG,Chunhua ZENG,Zhehua LI,Zhiqiang LI. Analysis of short-term temporal and spatial changes and sedimentary dynamics at the middle section of Haikou Bay Beach * [J]. Journal of Tropical Oceanography, 2019, 38(5): 77-85. |
| [15] | Xia WANG,Wendong FANG,Rongyu CHEN. Intra-seasonal variability of sea level anomalies and their propagation features in the northern South China Sea from 25 years of satellite altimetry data [J]. Journal of Tropical Oceanography, 2019, 38(3): 1-12. |
|
||