Journal of Tropical Oceanography ›› 2025, Vol. 44 ›› Issue (3): 157-166.doi: 10.11978/2024182CSTR: 32234.14.2024182
Previous Articles Next Articles
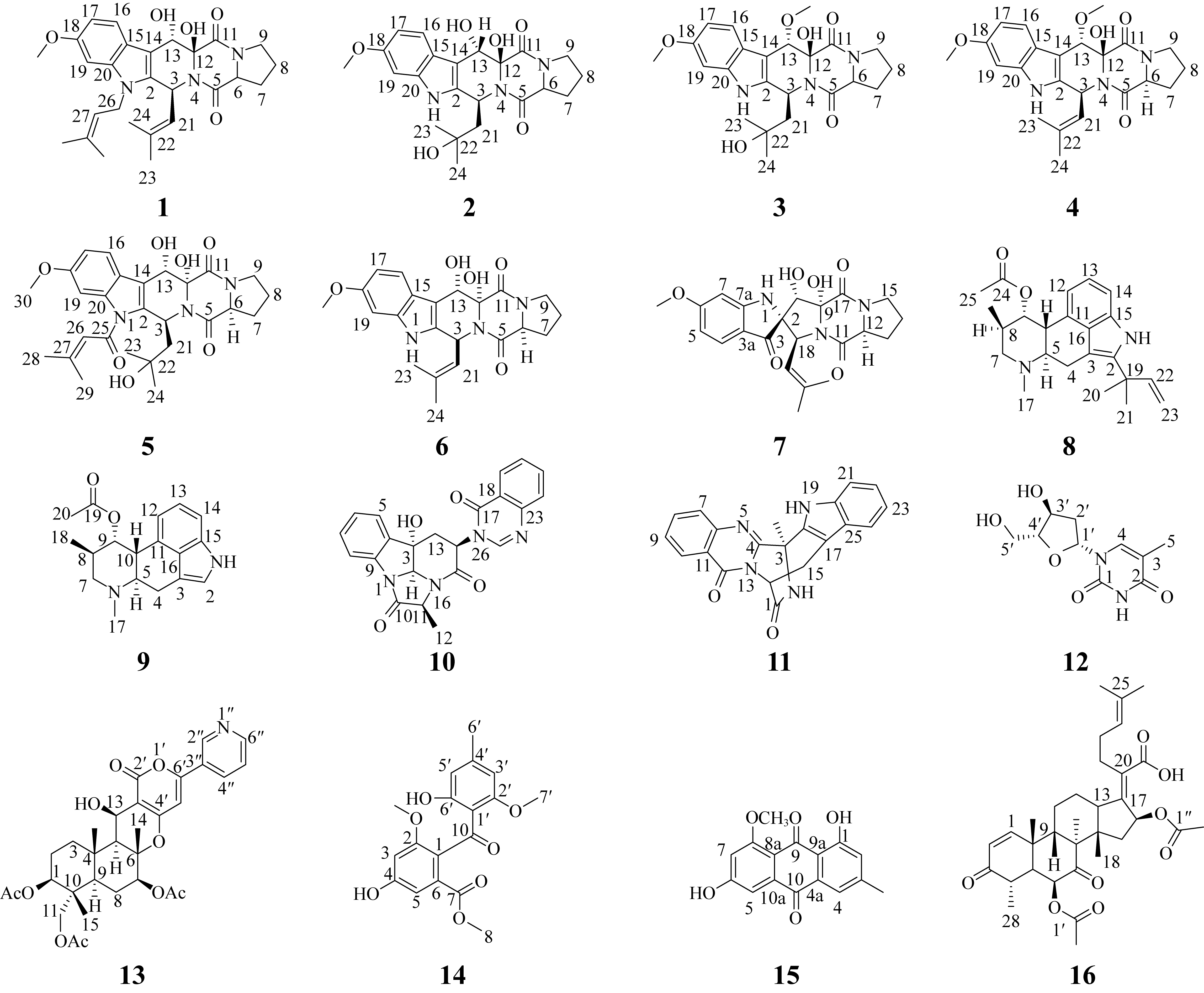
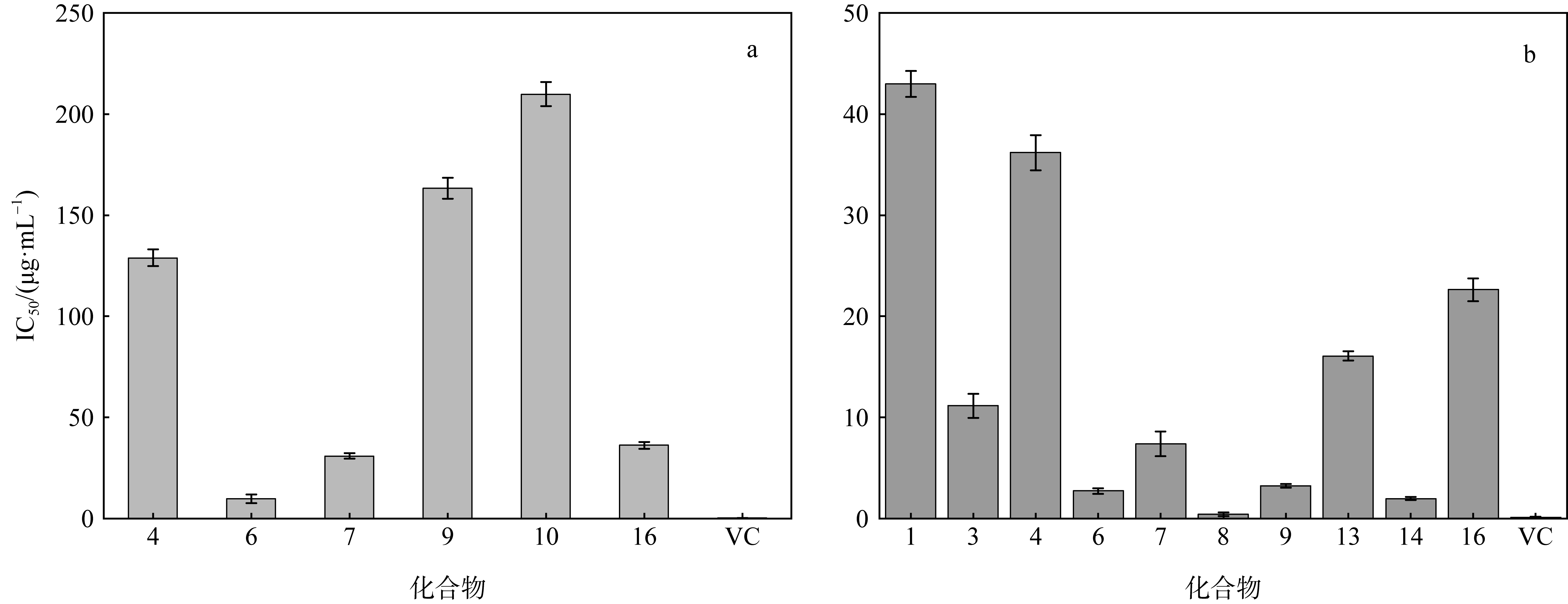
Research on the antioxidant and antibacterial activities of secondary metabolites from Gelidium-derived fungus Aspergillus fumigatus 9-1
HE Mingfeng( ), HUANG Jiaxiang(
), HUANG Jiaxiang( ), TIAN Yongqi(
), TIAN Yongqi( )
)
- College of Biological Science and Engineering, Fuzhou University, Fuzhou 350108, China
-
Received:2024-09-24Revised:2024-11-01Online:2025-05-10Published:2025-06-04 -
Contact:TIAN Yongqi -
Supported by:National Natural Science Foundation of China(42006094); Fujian Natural Science Foundation(2022J01561); Fujian Province-Indonesia Marine Food Joint Research and Development Center Open-Ended Foundation(Y1-KF2201)
CLC Number:
- Q936
Cite this article
HE Mingfeng, HUANG Jiaxiang, TIAN Yongqi. Research on the antioxidant and antibacterial activities of secondary metabolites from Gelidium-derived fungus Aspergillus fumigatus 9-1[J].Journal of Tropical Oceanography, 2025, 44(3): 157-166.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] |
丛梦静, 胡怡伟, 赵凯, 等, 2022. 深海冷泉来源真菌Talaromyces helicus SCSIO41311中聚酮类化学成分研究[J]. 热带海洋学报, 41(5): 117-120.
doi: 10.11978/2021168 |
|
|
|
| [2] |
冯婷, 孙建, 王玉妃, 等, 2024. 北部湾海洋真菌Aspergillus fumigatus DL-p0m-g2的化学成分及药理活性研究[J]. 热带海洋学报, 43(1): 154-166.
|
|
|
|
| [3] |
孔维琦, 金鑫, 刘永宏, 等, 2025. 羊栖菜共附生真菌Aspergillus sp. GXIMD 02045的次级代谢产物研究[J]. 热带海洋学报, 44(1): 154-161.
|
|
|
|
| [4] |
王小敏, 张民, 杨钰昆, 2015. 老蒜提取物萃取部位的抑菌活性及成分分析[J]. 现代食品科技, 31(1): 65-70.
|
|
|
|
| [5] |
姚佳晓, 贺小平, 周培娟, 等, 2019. 株海南三亚红树林真菌Alternaria alternate次级代谢产物的研究[J]. 中国海洋药物, 38(3): 64-70.
|
|
|
|
| [6] |
杨曦亮, 王健娇, 姜睿, 等, 2020. 海藻共附生真菌活性天然产物研究进展[J]. 广西科学, 27(5): 462-474.
|
|
|
|
| [7] |
张英格, 伍冠一, 陈曦, 2021. 石花菜化学成分与药理作用研究进展[J]. 海洋科学, 45(1): 129-138.
|
|
|
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
doi: 10.1021/acs.jnatprod.8b00382 pmid: 30070829 |
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
doi: S0308-8146(17)31984-2 pmid: 29329864 |
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [1] | YANG Jie, YAO Feihua, LI Xiaoyan, SHI Jieyu, YI Xiangxi, GAO Chenghai. Secondary metabolites from the fungal-bacterial symbiont Aspergillus spelaeus GXIMD 04541 / Sphingomonas echinoides GXIMD 04532 derived from Mauritia arabica [J]. Journal of Tropical Oceanography, 2025, 44(2): 56-63. |
| [2] | KONG Weiqi, JIN Xin, LIU Yonghong, GAO Chenghai, CHEN Xianqiang. Studies on secondary metabolites of Sargassum fusiforme derived fungus Aspergillus sp. GXIMD 02045 [J]. Journal of Tropical Oceanography, 2025, 44(1): 154-161. |
| [3] | XIAO Yang, YAN Jing, YANG Shuyu, ZHANG Tangjie, WENG Jie, LI Qilin, LIU Hongcun, MENG Juan, YANG Lifang. Study on the secondary metabolites and hypoglycemic and lipid lowering activities of actinomyces Streptomyces olivaceuss 10127 from the Guangxi mangrove sediments [J]. Journal of Tropical Oceanography, 2024, 43(6): 181-189. |
| [4] | YUAN Yuan, ZHANG Yuanyuan, XU Fuquan, LIU Qingchao, WANG Junfeng. Secondary metabolites from the sponge-derived fungus Aspergillus sp. SCSIO 41018 and their free radical scavenging activity [J]. Journal of Tropical Oceanography, 2024, 43(6): 196-200. |
| [5] | AN Fan, JIANG Yue, WANG Yu, CAO Guangping, GAO Chenghai, LIU Yonghong, YI Xiangxi, BAI Meng. Studies on secondary metabolites of endophytic fungus Aspergillus terreus GXIMD 03158 isolated from mangroves Acanthus ilicifolius L. [J]. Journal of Tropical Oceanography, 2024, 43(5): 41-48. |
| [6] | LIN Yingting, SUN Ying, HU Jiangnan, DONG Shuai. Study on secondary metabolites of the endophytic fungus Talaromyces sp. XXH006 Isolated from Conus literatus [J]. Journal of Tropical Oceanography, 2024, 43(4): 181-188. |
| [7] | WANG Jiaxi, LU Humu, QI Xin, GAO Chenghai, LIU Yonghong, LUO Xiaowei. Study on the secondary metabolites from the Weizhou Island coral Acropora austera associated fungus Arachniotus ruber GXIMD 02510 and their antibacterial activity [J]. Journal of Tropical Oceanography, 2024, 43(4): 174-180. |
| [8] | SUN Manman, ZENG Yanbo, XU Han, YAO Ligong, GUO Yuewei, SU Mingzhi. Chemical composition and antibacterial activities of the soft coral Lobophytum sp. from the South China Sea [J]. Journal of Tropical Oceanography, 2024, 43(4): 189-197. |
| [9] | QUAN Miaomiao, MA Qingyun, YANG Li, XIE Qingyi, DAI Haofu, HAO Yu’e, ZHAO Youxing. Study on the secondary metabolites of marine fungus Phaeospheriopsis sp. ZYX-Z-811 [J]. Journal of Tropical Oceanography, 2024, 43(2): 121-127. |
| [10] | LIU Ying, XIAO Yang, ZHU Zhenxin, LIU Hongcun, JIANG Mingguo, ZHU Yuzhang, LIN Kun, WU Jincheng, LU Xiaomei, HUANG Xiaoning, LIANG Haina, LU Wensen, YANG Lifang. Study on the Secondary Metabolites of Marine Streptomyces Sporoverrucosus 33510 [J]. Journal of Tropical Oceanography, 2024, 43(2): 128-134. |
| [11] | FENG Guangfu, HE Jeyi, BAI Meng, YI Xiangxi, LIU Xinming, LUO Lianxiang, GAO Chenghai. Secondary Metabolites from Hydaranth of Coral Goniopora columna in the Beibu Gulf [J]. Journal of Tropical Oceanography, 2024, 43(2): 135-139. |
| [12] | FENG Ting, SUN Jian, WANG Yufei, PAN Weibin, QIN Xucan, QIN Bingyun, ZHOU Liman, WANG Cong, WANG Pei, KONG Fandong. Study on the chemical constituents and pharmacological activity of the marine-derived fungus Aspergillus fumigatus DL-p0m-g2 in the Beibu Gulf [J]. Journal of Tropical Oceanography, 2024, 43(1): 154-166. |
| [13] | XING Nannan, REN Runxin, TANG Zhenzhou, LUO Zhihong, XIA Chenxi, LIU Yonghong, PENG Liang, CHEN Xianqiang. Study on the secondary metabolites of fungus Aspergillus sp. GXIMD02003 derived from marine sediment in the Weizhou island [J]. Journal of Tropical Oceanography, 2023, 42(5): 154-160. |
| [14] | ZENG Buyan, LIANG Zhifeng, LUO Qinqin, ZENG Ling, YANG Changgeng, WANG Liyun, SUN Yulin. Molecular identification, secondary metabolites and biological activities of a deep-sea-derived fungus 101#* [J]. Journal of Tropical Oceanography, 2023, 42(5): 104-114. |
| [15] | LIANG Hanqiao, CHEN Wenfeng, FAN Yikai, ZHU Zidong, MA Guoxu, CHEN Deli, TIAN Jing. Research progress on the secondary metabolites and activities of endophytic fungi of genus Aspergillus and Trichoderma from mangroves [J]. Journal of Tropical Oceanography, 2023, 42(4): 12-24. |
|
||